Chapter 5
From Cambridge to Vienna:
The Scintillation Counter in Female Hands
1Bringing money and instruments into an institute that is barely supporting its personnel might be necessary, but it is surely not sufficient for boosting it to the forefront of scientific research. The skills and the ingenuity of the experimenters are those elements that give life to the material culture of the discipline and often rework its theories. Hans Pettersson had both. He was keen in designing experiments, but he was also impulsive enough to have "big ideas"1 and persistent enough to pursue them. In a field that only "the Devil knows what can happen anytime," Pettersson was not afraid to play along.2
2In the early 1920s, the most challenging set of problems in radioactivity research was related to artificial disintegration. In 1919, Rutherford noticed an anomalous effect in the collision of alpha particles with nitrogen. When pure nitrogen was bombarded by fast radium C alpha particles, long-range atoms arose from the collision. Those were probably "atoms of hydrogen or atoms of mass 2. If this be the case," Rutherford argued, "we must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus."3
3In the fall of 1919, when Rutherford took over the directorship of the Cavendish Laboratory in Cambridge, he pursued his earlier studies on artificial disintegration with great zeal. As Jeff Hughes has documented, Rutherford reorganized the Cavendish Laboratory as a whole by introducing this new research program.4 An inflow of research students, changes in the material culture, and a spatial rearrangement of the laboratory marked his arrival in Cambridge. By the end of March 1920, Rutherford had concluded that the particles from nitrogen were actually hydrogen nuclei, as he had first speculated.5
4In the course of 1921, Rutherford tested a series of light elements for the disintegration phenomenon with the help of James Chadwick, his research student in Manchester. Chadwick had followed him to Cambridge, serving as a reliable and experienced experimenter in his team. As they both concluded, only these elements whose atomic mass was given by 4n + 2 or 4n + 3 where n was a whole number, expelled long-range disintegration particles. Seventeen other elements, including carbon and oxygen, yielded no detectable disintegration protons. In August 1921, Rutherford and Chadwick thus argued that the atomic nucleus consisted of a central core of alpha particles surrounded by protons as distant satellites.6 The artificial disintegration experiments had a theoretical bearing that made them attractive to any ambitious researcher. Pettersson was undoubtedly one of those.
5  Gerhard
Kirsch at the Radium Institute, c 1925. Shortly after his first visit to Vienna in 1922,
Pettersson established a close collaboration with Gerhard Kirsch and worked on
artificial disintegration. Their first paper came out
in 1923 and a full version was published in the Sitzungsberichte of the Vienna
Academy and in the Philosophical Magazine, while a short report
appeared also in Nature.7 The multiple publications showed the importance of their results, which differed
significantly from those obtained in Cambridge. "Our results," as
they both argued, "seem so far to indicate that the hydrogen nucleus is a
more common constituent of the lighter atoms than one has hitherto been inclined
to believe."8 Elements such as beryllium, magnesium, and silicon were disintegratable despite
the fact that Rutherford and Chadwick had stated otherwise. With that paper,
Pettersson and Kirsch generated a milestone in the history of physics controversy
with Rutherford's research group, one that historian Roger Stuewer has
documented in detail.
Gerhard
Kirsch at the Radium Institute, c 1925. Shortly after his first visit to Vienna in 1922,
Pettersson established a close collaboration with Gerhard Kirsch and worked on
artificial disintegration. Their first paper came out
in 1923 and a full version was published in the Sitzungsberichte of the Vienna
Academy and in the Philosophical Magazine, while a short report
appeared also in Nature.7 The multiple publications showed the importance of their results, which differed
significantly from those obtained in Cambridge. "Our results," as
they both argued, "seem so far to indicate that the hydrogen nucleus is a
more common constituent of the lighter atoms than one has hitherto been inclined
to believe."8 Elements such as beryllium, magnesium, and silicon were disintegratable despite
the fact that Rutherford and Chadwick had stated otherwise. With that paper,
Pettersson and Kirsch generated a milestone in the history of physics controversy
with Rutherford's research group, one that historian Roger Stuewer has
documented in detail.
6Between 1923 and 1924, the debate got heated, with the two groups zealously publishing their conflicting experimental results in the most internationally prestigious journals. At stake was not only the authority of the Cavendish Laboratory in the world of radioactivity and Rutherford's theoretical satellite model. The material culture of the Cambridge group—its experimental methods, the instruments, and the politics of collaboration they embodied—were under vigorous attack. The focus was mainly on the scintillation counter.
7Considering the scintillation counter as a historical document, one can see that for both groups, it reflected a number of social and epistemic meanings embodied in its construction and use.9 With Vienna being the major link that tied together a small group of physicists and chemists, the scintillation counter enabled them to bring their laboratory to the cutting edge of radioactivity research. The scientific knowledge required for its use further sustained the work of a number of women in the institute and brought together scientists from different research teams. Demonstrating technical dexterity, female physicists manipulated the counter and played the role of instrument makers along with their male colleagues.
8Still, scientific instrument makers have not been studied in their institutional context.10 The rise of practical scientific research in the nineteenth and early twentieth centuries led to an increasing demand for specialized scientific instruments. Special contractual arrangements with local makers, the direct employment of skilled technicians on the staff of the scientific institutions, and the commercial supply from instrument-making companies satisfied those needs. In Vienna, men and women often played the role of instrument maker, saving money for the institute. Focusing on their contributions meant paying attention on how experiments were performed and how those scientists formed their identities as experimenters while actively engaged in all aspects of the experiment. In such cases, women's contributions are rarely mentioned, nonetheless.
9Despite the vast literature on technology and gender, most recent studies have focused on biomedical and information technologies.11 When the physics laboratory is addressed, the point is to demonstrate that women are often used as calculators, scanners, unskilled assistants, and cheap labor force in science.12 The active role of female researchers at Vienna's Radium Institute, in contrast to the Cambridge group, reveals implicit assumptions about gender roles within the institute, explicit gender politics of collaboration, and most importantly institutional differences in the relation of gender and laboratory technologies.
The Early Days of the Scintillation Counter
10  Crooke's
spinthariscope. What for Friedrich Giesel was the best way to
detect the easily absorbed alpha radiation of polonium, became for William
Crookes the best way to exhibit the luminosity of radium on a screen. We have
already encountered Giesel when he handed the first amount of radium to Meyer
back in 1899. Crookes was an exceptionally gifted chemist interested in "those
areas where chemistry meets physics."13 When he approached Giesel and asked him to recommend a medium for exhibiting
radium on a screen, Giesel suggested zinc sulfide.14 In 1903, Crookes became the first to describe the evanescent flashes appearing
on the suggested screen when he brought radium close to it. Performing several
experiments in order to test different factors influencing the number of
scintillations, Crookes constructed a very simple apparatus. No schematic
representation accompanied his publication and only a short description was
given: "A blend screen was fixed near a flat glass window in a vacuum tube
and a piece of radium salt was attached to an iron rocker so that the movement
of an outside magnet could bring the radium close to the screen or draw it away
altogether. A microscope gave a good image of the surface of the screen and in
a dark room, the scintillations were well seen."15 The first scintillation counter was already constructed and Crookes proposed to
call the instrument a "spinthariscope" from the Greek word
spintharis—a scintillation.16
Crooke's
spinthariscope. What for Friedrich Giesel was the best way to
detect the easily absorbed alpha radiation of polonium, became for William
Crookes the best way to exhibit the luminosity of radium on a screen. We have
already encountered Giesel when he handed the first amount of radium to Meyer
back in 1899. Crookes was an exceptionally gifted chemist interested in "those
areas where chemistry meets physics."13 When he approached Giesel and asked him to recommend a medium for exhibiting
radium on a screen, Giesel suggested zinc sulfide.14 In 1903, Crookes became the first to describe the evanescent flashes appearing
on the suggested screen when he brought radium close to it. Performing several
experiments in order to test different factors influencing the number of
scintillations, Crookes constructed a very simple apparatus. No schematic
representation accompanied his publication and only a short description was
given: "A blend screen was fixed near a flat glass window in a vacuum tube
and a piece of radium salt was attached to an iron rocker so that the movement
of an outside magnet could bring the radium close to the screen or draw it away
altogether. A microscope gave a good image of the surface of the screen and in
a dark room, the scintillations were well seen."15 The first scintillation counter was already constructed and Crookes proposed to
call the instrument a "spinthariscope" from the Greek word
spintharis—a scintillation.16
11What Crookes thought he was actually observing was the impact of electrons on the screen. Shortly after, Julius Elster and Hans Geitel confirmed Crookes' observations. In 1908, Erich Regener used the method to record alpha particles of polonium.17 In his Nobel speech that same year, Ernest Rutherford compared the scintillation technique to the electrical method for counting alpha particles that he and Hans Geiger had constructed. The scintillation counter was found to be reliable. He further proved that each alpha particle produced a single visible scintillation on the screen.18 While Rutherford was still in Manchester, his assistants Walter Makower and Geiger set up a course in training research students in the experimental techniques used in radioactivity. The scintillation method was by far the most important.19
12In 1919, manipulating the counter for his collision experiments, Rutherford argued, "Under good conditions, counting experiments are quite reliable from day to day." Taking personal charge of the counting, he added, "Those [countings] obtained by my assistant Mr. W. Kay and myself were always in excellent accord under the most varied conditions."20 In his Bakerian lecture in 1920, where Rutherford proposed his satellite model of the nucleus, it was apparent that he relied on the use of the scintillation method for his artificial disintegration experiments.21 Placing great value on the technique and especially on the scintillation observers, Rutherford never forgot to acknowledge them in his papers. For William Kay, he granted an important role to the discovery of artificial disintegration by thanking him "for his invaluable assistance in counting scintillations."22
The Use of the Scintillation Counter in Cambridge
13In the early 1920s, the scintillation counter in its generic form was a very simple instrument. It consisted of a screen, a thin glass plate spread with an equally thin layer of zinc sulfide. When it was struck by charged particles, the screen produced light flashes. The scintillations were observed through a microscope which was specifically designed to increase the brightness of the flashes. By manipulating the microscope and its light-gathering power, the experimenter could work with weak radioactive sources and still observe a fair number of particles. A typical scintillation counting experiment could proceed as follows:
There must be two rooms and two workers. One of the rooms must be kept in good deal darker than a photographic dark room, and in it there is one of the men who is to act as the observer . . . In this room there is a microscope and scintillation screen, and also whatever may be set up of radium appropriate to the experiment. In the neighboring room the other sits and keeps the record of the count of the scintillations. Thus it may be that what is to be counted is the total number of scintillations made on the screen in two minutes and at the end of it he will write down the number told him by the observer. When the experimental set-up is to be changed, the [observer] must first blind-fold himself, and then the light is put on in the dark room and the other man comes in and resets the instrument. He must turn out the light and go out and shut the door before the observer can uncover his eyes. Altogether it is a laborious business.23
14Indeed, the detection of alpha particles in zinc sulfide screens by the visual observation of the individual scintillations was neither easy nor always reliable. The evidence was fragile, depending on the radioactive contamination of the counting apparatus, on the experience of the observer, and on the presence of hydrogen impurities. Besides alpha particles, the source often produced beta and gamma radiation that interfered with counting and increased the number of flashes. Additionally, weak scintillations were not always observable. The observer needed to have control not only of the counter but of his optical system as well. Rutherford, Chadwick, and Ellis affirmed this: "The superior efficiency of an experienced observer appears to be due to greater concentration, to control spontaneous movements of the eye, and to practice using external portions of the retina, thereby avoiding the insensitive fovea-centralis."24 The issue of control stood at the center of Rutherford's research project at Cavendish in a dual sense. Control of the observers of the scintillations meant constant crosschecks of their countings and manipulation of their apparatus. To Rutherford, control had a further bearing on his entire laboratory life. Essential to his theoretical atomic model, the scintillation counter was the means by which Rutherford hoped to detect the atomic structure.
15Rutherford enlisted his students "under Chadwick's careful surveillance,"25 and he eventually assigned specific research projects to them after a short introductory course which included training in scintillation counting. The aim was to complete a Ph.D. in three years, contributing mostly to the laboratory's research and problem-solving tradition. With Rutherford's research program depending entirely on the scintillation counter, the modification of the technique became urgent.
16The first step was to change the optical system by utilizing a new microscope that increased the brightness of weak scintillations. "We have found most suitable for our purpose a holoscopic objective of focal length 16mm and aperture 0.45."26 The final magnification of the system was about 40. By choosing wide aperture, the scintillations became more brilliant and counting became easier. They also tried to obtain more powerful sources of radiation. Radium was at Rutherford's "command," a trafficking material he brought with him from Manchester.27 Furthermore, to protect the observer from the gamma rays of the source, Rutherford and Chadwick used suitable absorbing screens and a reflecting prism. Next, they improved the scintillation screen by employing a thinner and finely powdered layer of zinc sulfide, aiming to reduce the luminosity due to gamma rays. In addition, a strong magnetic field deflected the beta rays. It was at this point that the scintillation counter developed from a clumsy technology to Rutherford's powerful vehicle for restructuring not only the material culture of his laboratory but physics in the Cavendish Laboratory as well.28 In the hands of the Cambridge experimenters, the scintillation counter was transformed into a promising technique for maintaining the laboratory's authority in the postwar world of radioactivity.
17The above changes in the material structure of the instrument simultaneously posed constraints that altered the politics of collaboration in conducting experiments. To protect against visual mistakes and exhaustion, male researchers alternated in counting scintillations for one minute each. Indeed, as Rutherford's acknowledgments show, the practice of scintillation counting in Cambridge was a male preserve and this included the task of recording data, making the necessary adjustments to the instrument, and, most importantly, observing evanescent scintillations.
18Likewise, in Rutherford's group, the tasks of instrument-making and experimenting were also gendered. From the published historical studies on Rutherford's team, it appears that there were no female researchers participating in the artificial disintegration experiments. In Manchester, Rutherford had a number of female research students, but none in Cambridge.29 In the original papers of Rutherford and Chadwick, there were no female research students mentioned as scintillation counters. However, the papers often thanked men such as Ellis, Blackett, Barton, Hirst, and Osgood. As Heilbron suggests, Rutherford's laboratory was "a masculine and, sometimes, a macho place." Rutherford himself referred to his task as a professor as "driving the boys along" although he had no objection to "a woman of great charm and ability . . . a welcome addition to any research laboratory."30
19The Cavendish Laboratory was established in 1874 to teach the physical sciences to male students of mathematics. Despite their actively pursued petitions, women had been consistently refused degrees from the University of Cambridge both by its authorities and by its male undergraduates who feared competition for jobs and loss of freedom. The refusal of full degrees and membership was the reason for women to establish their own colleges, one in Girton outside Cambridge (1873) and one on the edge of Newnham Hall (1875). Although the two colleges were in no way considered part of the university, women had the privilege to receive some of the best academic training available through the university and take Tripos exams on an unofficial basis. In 1882 following a suggestion by Lord Rayleigh, it was decided that all classes and demonstrations should be opened to the students of the two women's colleges, including the Cavendish Laboratory. Paula Gould has concentrated on those women who entered the laboratory as research students in the late nineteenth century, arguing that they "were not isolated oddballs, figures of fun for discussion by the male workers. They were family friends, relations, educated women, keen researchers seeking experimental space."31
20Despite having the educational advantages similar to their male colleagues, women did not gain formal university acceptance until the early 1920s. A Royal Commission was set up in 1919 to review major changes due to the war years and to recommend new policies, including women's status in Cambridge. On October 20 of that year, a grace giving women the titles of degrees with no participation in the university's government was passed. Thus, at the time Rutherford took up his professorship at Cambridge, the situation of women was very much improved. This included first a new regulation in 1923 which allowed women to have full access to the university library and borrow books and second the decision of the Royal Commission to leave it to the university itself to decide on the admission of women to full membership.
21Although Rutherford favored women's full equality with men, Joseph John Thomson was in favor of maintaining a few restrictions. It is indicative that the 1921 group photographs of the Cavendish researchers show one woman out of 29 people, one out of 25 people in 1923, and two out of 39 in 1932.32 The Cavendish Laboratory experiments, including the scintillation counter and the observation of flashes were apparently male projects. They embodied specific politics of collaboration and illustrated gender assumptions about skill in observing and manipulating the apparatus.
Technology Transfer: The Scintillation Counter in Vienna
22  Hans
Pettersson at the Radium Institute, c 1925. In late 1922, when Pettersson and Kirsch performed
their first artificial disintegration experiments at the Radium Institute in Vienna,
they used the scintillation method exclusively. If they were to undermine
Rutherford's and Chadwick's experimental results concerning the artificial
disintegration of light elements, there was no alternative to the scintillation
technique.33 However, as Galison has illustrated, "Objects travel clothed in culture
and human interactions. Objects are encumbered, covered with meanings,
symbolisms, power, and the ability to represent but also to preserve specific
elements of continuity. Yet, precisely because things come dressed with
meaning, it is essential not to picture the handing down as occurring without
alternation."34 Through its transfer from Cambridge to Vienna, the scintillation counter was
transformed into a different instrument, "clothed" now in the Viennese culture
of radioactivity. It is those transformations I want to trace here, focusing
especially on the alteration of the gender assumptions that the apparatus
carried to the Radium Institute.
Hans
Pettersson at the Radium Institute, c 1925. In late 1922, when Pettersson and Kirsch performed
their first artificial disintegration experiments at the Radium Institute in Vienna,
they used the scintillation method exclusively. If they were to undermine
Rutherford's and Chadwick's experimental results concerning the artificial
disintegration of light elements, there was no alternative to the scintillation
technique.33 However, as Galison has illustrated, "Objects travel clothed in culture
and human interactions. Objects are encumbered, covered with meanings,
symbolisms, power, and the ability to represent but also to preserve specific
elements of continuity. Yet, precisely because things come dressed with
meaning, it is essential not to picture the handing down as occurring without
alternation."34 Through its transfer from Cambridge to Vienna, the scintillation counter was
transformed into a different instrument, "clothed" now in the Viennese culture
of radioactivity. It is those transformations I want to trace here, focusing
especially on the alteration of the gender assumptions that the apparatus
carried to the Radium Institute.
23As a gifted instrument maker, Pettersson's first step was to improve the preparation of the radioactive source employed in the experiments. He developed a new method for the preparation of radium C that produced alpha particles of high intensity. The method involved the enclosure of dry radium emanation mixed with pure oxygen in thin-walled capillary tubes made out of quartz.35 Later, with the help of Kirsch, Pettersson constructed a different emanation vessel "in which the substances to be examined are spread in thin layers over copper."36 According to their results, silicon, beryllium, magnesium, and lithium yielded protons of ranges 18 cm, 12 cm, 13 cm, and 10 cm in air, respectively. Apparently contradicting Rutherford's and Chadwick's research, those elements proved to be disintegratable.
24In the late autumn of 1922, two of Rutherford's students, L. Bates and J. Rogers, were assigned to study long-range alpha particles from radium C. When Pettersson and Kirsch published their first paper, Bates and Rogers already had a response in hand. On September 22, 1923, they reported in Nature that radium C emits not only the usual range alpha particles of 7 cm but also ones of longer ranges.37 Thus, they argued, it could have been possible that what Pettersson and Kirsch thought they were observing were actually long-range alpha particles instead of disintegration protons.
25In their reply, Pettersson and Kirsch stressed the fact that the ratio in luminosity between alpha and H-particles (protons) does not permit such a mistake as Bates and Rogers attributed to them.38 On July 19, even before the articles were published, Rutherford sent his results to Pettersson.39 In a friendly and grateful response on July 27, Pettersson tried to reconcile and explain the discrepancies. His line of defense was centered on the scintillation method that he and Kirsch had already modified:
I lately had the counting box modified so as to allow the source as well as the substance of being enclosed while the pressure is varied. Preliminary experiments seem to show that with this arrangement, contamination can be avoided also at low pressures, so that the particles from carbon and other elements may be investigated down to the very shortest ranges. 40
The modification of the counting box was not the only innovation that the Viennese experimenters added to the scintillation counter. As Pettersson said:
Our newest microscope with the scintillation screen directly attached to the front lens by means of cedar oil (a Watson Holoscopic of n.a. 0.70 and f=12) is so superior with regard to brilliancy of the scintillations viewed through it, we feel much more confident than with the microscope previously used not only in differentiating between scintillations from alpha and from H-particles but also in counting the latter relatively near the end of their range. For this reason alone, I regard any confusion between H-particles and contamination alpha-particles as improbable.41
26In their next publication, submitted to the Proceedings of the Royal Society on December 5, 1923, Bates and Rogers seemed doubtful. Without any direct reference to Pettersson and Kirsch, they suggested that the observed long-range particles were alpha particles from the contamination of the source. However, they admitted that "this evidence is far from conclusive, as these particles have never been observed alone but always when accompanied by particles of different types."42 Faithful to the material culture of their laboratory, they used the same microscope with a holoscopic objective of numerical aperture 0.45 and 16 mm focal length that was previously used by Rutherford and Chadwick.
27Specially constructed, the eyepiece consisted of two large plano-convex lenses and a smaller double convex eye lens. Such arrangements resulted in increasing substantially the field of view. Between the lines was an implicit attack to the microscope used in Vienna. By having a smaller field of view in their microscope, Pettersson and Kirsch were forced to increase the intensity of the source and thus the secondary radiation. Somewhat regretful, Bates and Rogers pointed out that "the scintillation method is the only method at present available for investigating these long-range particles."43 They avoided any reference to the Viennese group when, in February 1924, they sent their next study to the Proceedings of the Royal Society. More confident this time, Bates and Rogers argued that the long-range particles described in their experiment were emitted by the source itself.44 Additionally, they had slightly altered the scintillation method by introducing polonium as the radioactive source instead of radium C. There was the significant advantage that polonium did not emit beta rays.
28The task of preparing polonium was neither easy nor common knowledge among the experimenters. Chadwick, as a senior colleague and greatly interested in the project, prepared the polonium source for Bates and Rogers from a solution of radium D.45 Eventually, the contamination of the source with radium forced the two research students to use instead the contents of old emanation tubes provided by Rutherford.
29While Rutherford enlisted in his group research students who never forgot to acknowledge his support, Pettersson enrolled more experienced experimentalists.46 Dagmar Pettersson entered the debate on April 3, 1924, presenting her first paper to a meeting in the Vienna Academy.47 She brought to the Radium Institute technical knowledge and the chemical expertise of her recent work at the Technische Hochschule, as well as the experience she had gained working with Otto and Hans Pettersson.
Dagmar Wendel-Pettersson
30  Dagmar
Pettersson at the Radium Institute, c 1925. Daughter of a prosperous civil
engineer, Dagmar Pettersson, née Wendel, received private education and entered
the University of Uppsala to study chemistry as her major and mathematics as her
minor.48 All of Dagmar's three sisters sought professional training, prompted by their
father to ensure a self-supporting life. Dagmar was the eldest, born
in 1888. She finished her studies in 1914 and soon after secured a position as
a chemist in an agricultural laboratory in Skenja, Sweden, playing a leading role in building
a new chemistry lab.49 Shortly after she sought a new position, wishing to return to Göteborg and be
closer to her parents.
Dagmar
Pettersson at the Radium Institute, c 1925. Daughter of a prosperous civil
engineer, Dagmar Pettersson, née Wendel, received private education and entered
the University of Uppsala to study chemistry as her major and mathematics as her
minor.48 All of Dagmar's three sisters sought professional training, prompted by their
father to ensure a self-supporting life. Dagmar was the eldest, born
in 1888. She finished her studies in 1914 and soon after secured a position as
a chemist in an agricultural laboratory in Skenja, Sweden, playing a leading role in building
a new chemistry lab.49 Shortly after she sought a new position, wishing to return to Göteborg and be
closer to her parents.
31In the first decade of the nineteenth century, women who sought careers in science in Sweden were very few and quite visible. Dagmar had met Hans Pettersson when both were students in Uppsala, but it was not until her return to Göteborg that they developed a relationship. Hans offered to help by asking his father to hire Dagmar as a chemist in his oceanographic station in Bornö. Otto already had a female chemist assistant and seemed to have been fairly open in accepting women in his lab.50
32After his son's intervention, Otto employed Dagmar to measure the salinity of deep water samples as a research assistant at Börno. Two years later in 1917, Hans and Dagmar were married and when he moved to Monaco, she joined him not only as his wife but as a colleague with considerable experience in chemistry.51 When they moved to Vienna in late 1922, she secured a position at the Technische Hochschule, Vienna's Polytechnic, working in a lab as a chemist and conducting research at the Radium Institute in support of Hans's project.
33Although their 3-year-old daughter joined them in Vienna in 1924, Dagmar was able to continue her work. Her tasks were not just supplementary to her husband's research. As her diary indicates, she was involved in designing apparatus for the groups experiments and her own, counting scintillations, extracting polonium from residues from Joachimsthal radium ores, and critiquing Hans's manuscript. The fact that her husband was a scientist probably made it easier for her to combine motherhood with her scientific research.52
34Directly addressing Bates's and Rogers's experiments, Dagmar attempted to undermine their results by altering the scintillation method. Instead of allowing alpha particles to pass through air or mica as in the Bates and Rogers experiments, Dagmar enclosed the scintillation counter in a glass tube. She constructed a device for sending alpha particles emitted from radium C to the scintillation screen through thin foils of gold or of copper which acted as primary absorbers for the ordinary a-particles.53
35 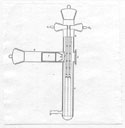 Dagmar Pettersson's
scintillation counter. Dagmar's apparatus
consisted of a glass t-tube that directed the alpha-particles emitted from the
source to the scintillation screen (Z) to pass through the carrier of the
radioactive preparation (T). The gold absorbing foil (F) was placed in front of
the preparation (P) in order to minimize the risks of getting secondary
particles other than those of the source. Additionally, mica screens of varied
stopping powers were interposed between the scintillation screen and the
source. The mica screens offered the advantage of placing weak radioactive
sources closer to the scintillation screen. Two frames (M1) and (M2)
with five openings each, hanging from (G1) and (G2)
vertically in the tube, determined the different absorption levels from 0 to 16
cm equivalent of air. The pipe (H) was designed to control the pressure in the
tube and a magnetic field deflected the beta rays emitted from the source.
Dagmar Pettersson's
scintillation counter. Dagmar's apparatus
consisted of a glass t-tube that directed the alpha-particles emitted from the
source to the scintillation screen (Z) to pass through the carrier of the
radioactive preparation (T). The gold absorbing foil (F) was placed in front of
the preparation (P) in order to minimize the risks of getting secondary
particles other than those of the source. Additionally, mica screens of varied
stopping powers were interposed between the scintillation screen and the
source. The mica screens offered the advantage of placing weak radioactive
sources closer to the scintillation screen. Two frames (M1) and (M2)
with five openings each, hanging from (G1) and (G2)
vertically in the tube, determined the different absorption levels from 0 to 16
cm equivalent of air. The pipe (H) was designed to control the pressure in the
tube and a magnetic field deflected the beta rays emitted from the source.
36For the preparation of radium C, Dagmar adopted Pettersson's innovative method of using thin capillary tubes filled with radium emanation and dry oxygen. The microscope used for the observation of the light flashes was, as Dagmar argued, superior to that of Rutherford's and Chadwick's. That gave her the opportunity to observe the slow, weak scintillations from H-particles. Used previously by Kirsch and Pettersson, the microscope was directly attached to the scintillation screen. It had a numerical aperture of 0.70 and the field of view was decreased from 12.5 mm2 to 8 mm2.54 "It may be added," Dagmar argued, "that the use of a greatly improved microscope made it relatively easy to distinguish between scintillations from H and from alpha particles, so that they could be counted separately."55 According to the final results, the number of alpha particles of range more than 9.2 was, to the surprise of Bates and Rogers, zero. That meant that there were no long-range alpha particles emitted from the source. Dagmar published her relevant article not only in the Mitteilungen of the institute but also in Nature.56
37Dagmar's first ambitious articles in the world of radioactivity research were actually her last ones as well. Although she continued to work in Pettersson's group, Dagmar did not publish and, according to the recollections of her daughter, "Research work never was prominent for my mother."57 Shortly after her publication, Rutherford and Chadwick challenged her observations, criticizing both her experimental setup and her apparatus. As they argued, it was probably the use of the absorbing foils that misled Dagmar into detecting the long-range alpha particles.58 But her claims had clearly made an impression in Cambridge. Much later, in a letter to his father, Pettersson described Bates' interesting account of the episode:
I was visited by my enemy, nowadays friend, former colleague of Rutherford, now with Porter in University College where I met him in May. He described very dramatically how Dagmar's letter to Nature—in which she opposed the results of Bates and Rogers—had hit the Cavendish laboratory like a bomb and how B[ates] had been scolded by R[utherford] in spite of his being right to certain degrees.59
38In preparing her experiments, Dagmar collaborated closely with a young lady, giving rise to Pettersson's later claims for the "unselfish" and collegial ethos of his group.60 "I thank Elisabeth Kara-Michailova for the zinc sulfide screen," Dagmar acknowledged, "which she produced through careful examination of different scintillation substances and methods of preparation."61
39At the time, Kara-Michailova was 27 years old with a short but important list of publications already in her vita. At the academy meeting on May 8, 1924, Kara-Michailova presented a new technique that she and Hans Pettersson had developed for identifying the flashes between alpha and H-particles.62 Their aim was essentially to reshape the scintillation counter by utilizing a new microscope they had constructed. The result was to deeply alter not only the counter but also the gender assumptions that accompanied its use. Where Cambridge researchers aimed for a well-defined hierarchical division of labor among the experimenters, the Viennese chose a collegial partnership. It was under these assumptions that Kara-Michailova teamed up with Pettersson in 1923.
The Kara-Michailova/Pettersson Collaboration
40By the mid 1920s, although both the Vienna and the Cambridge groups had immensely improved the scintillation counter, its main disadvantage continued to be the difficulty of distinguishing between flashes produced by different kinds of particles. As Pettersson and Kirsch suggested, the considerable differences in the relative brightness of scintillation from alpha and H-particles required more attention and exact measurements.63 Having an expertise in measurements of luminescence, Kara-Michailova was the most appropriate collaborator for Pettersson.
41In early 1924, with the help of his Swedish sponsors, Pettersson purchased a so-called Vergleichsokular (a comparison eyepiece) from the C. Reichert Company in Vienna.64 The eyepiece was designed to compare the images from two microscopes in combination with two Watson holoscopic objectives. Pettersson estimated the cost of a Watson scintillation microscope, a comparison eye piece for scintillation photometry, two holoscopic objectives, and a number of scintillation screens and absorbing foils, to be 86 dollars, a considerable amount at the time.65 In the Radium Institute, there was no previous experimental tradition with such an optical system. As a result, Pettersson purchased the whole set, altering not only the scintillation method but the material culture of the institute as well.
42 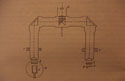 The scintillation counter used by Elisabeth
Kara-Michailova and Hans Pettersson. As this figure
shows, FOF was the Vergleichsokular, with two identical microscopes directly
built to the same eyepiece. Those were Watson holoscopic objectives with
numerical aperture 0.45 each and focal length 16 mm. S was the fixture holding
a reflective prism that offered the opportunity to combine the pictures from
both microscopes or selectively reflect the picture from only one. The
microscope on the left was set to observe the scintillations produced by
H-particles on the screen (Z). The source (H) was either radium emanation or
radium C and the Leyboldt magnet (M) was set to deflect the beta rays of the source.
The microscope on the right was set to observe the flashes of alpha particles
produced by a polonium preparation (P). The zinc sulfide screens scintillated
in response to both kinds of particles, but the relative brightness of the
flashes was determined by means of light-absorbing screens (F) of known
absorption.
The scintillation counter used by Elisabeth
Kara-Michailova and Hans Pettersson. As this figure
shows, FOF was the Vergleichsokular, with two identical microscopes directly
built to the same eyepiece. Those were Watson holoscopic objectives with
numerical aperture 0.45 each and focal length 16 mm. S was the fixture holding
a reflective prism that offered the opportunity to combine the pictures from
both microscopes or selectively reflect the picture from only one. The
microscope on the left was set to observe the scintillations produced by
H-particles on the screen (Z). The source (H) was either radium emanation or
radium C and the Leyboldt magnet (M) was set to deflect the beta rays of the source.
The microscope on the right was set to observe the flashes of alpha particles
produced by a polonium preparation (P). The zinc sulfide screens scintillated
in response to both kinds of particles, but the relative brightness of the
flashes was determined by means of light-absorbing screens (F) of known
absorption.
43In the flow of their main text, Pettersson and Kara-Michailova did not forget to acknowledge the help of Maria Belar in measuring the absorption of the gray glasses (F). "We thank Maria Belar for the measurement of the absorption of the individual gray glasses that she conducted by the means of a Glan spectrophotometer in yellow-green light."66 At that time, Belar was closely working with Przibram and had just finished her thesis on the spectrophotometric method.67 The same year, Kara-Michailova also published an article in the prestigious Physichalische Zeitschrift on the scintillation method and the quantitative optical differentiation between alpha and H-particles.68 In the autumn of 1925, Kara-Michailova was forced to quit her research for a few months. Suffering from a lung infection, she moved to Merran in Tirol to recover.69 As her publication record indicates, that interruption cost her more than a year of active work although she actually returned to the institute in the spring of 1926. Their project was by no means on secure footing and thus Pettersson was in urgent need of financial support.
Funding from the Rockefeller Foundation
44"I have the pleasure to state that, in my opinion, the method developed by Doctor Pettersson and his coworkers is of great scientific value and that the pursuance of this scientific work merits every assistance," Albert Einstein said of Pettersson's work in 1923.70 The next year Pettersson tried to approach the International Education Board (IEB), securing again Einstein's recommendation.71 The immediate response of the board was negative, but its officials were intrigued. On March 26, 1925, Augustus Trowbridge, director of the European Physical and Biological Sciences Division of the IEB, visited Vienna to meet with Meyer, Przibram, and the director of the Austrian Academy of Sciences to evaluate the situation.
45Obviously, IEB could not support an institute without having a hand in it. In his report, Trowbridge first evaluated the status of the institute and its finances to Wickliffe Rose, the president of the IEB. From the institute's prewar glory, only the infrastructure and the expensive radium sources remained, but there was no soft money for research. Przibram was explicit in stating that no money was needed for assistants. Paradoxically, desperately needed were the cheapest accessories because breakable material could not be easily replaced. As Trowbridge realized, young researchers were working under "what an Occidental scientist could consider to be impossible working conditions through the lack of the most necessary materials and supplies." The main contributions came from sources that Pettersson solicited. Then, Trowbridge made his suggestion:
In my opinion, were the board to vote yearly, for one or two years, one fifth of this sum to the Vienna Academy of Sciences for the purpose of the purchase of necessary supplies and apparatus for research, this excellent institute could continue to train effectively more than a dozen young research students in the physical sciences and bring them to the point where a few of the best might then be given fellowships for further study in the west of Europe or America . . . . The Radium Institute of Vienna ought to be a natural feeder from southeastern Europe to the laboratories of Madame Curie and Sir Ernest Rutherford.72
46The International Education Board's choices in funding profoundly affected and shaped physics research in Europe. The IEB was created in 1923 as the Rockefeller Foundation's education board, with a mission both to provide fellowships for young scientists internationally and to support the infrastructure of science through capital grants for building and maintaining research institutes. The unstable political situation and the financial problems that European researchers were facing made the IEB a major actor in determining physicists' careers and the future of research programs.73 Likewise, the future of Pettersson's research group in Vienna and his own career depended substantially on the plans and decisions of the board. While IEB was willing to help the Radium Institute, their direct interest lay in the two other important radioactivity research centers in Paris and Cambridge and they viewed Vienna as their supplier of well-trained personnel. They never envisioned Vienna as an independent center capable of being in the forefront of research.
47On May 29, 1925, the board decided to offer the Radium Institute 2,000 dollars per annum for a period of not more than three years for the purchase of necessary equipment. The grant was put in the hands of the Austrian Academy of Sciences on July 1, 1925.74 In the autumn of the same year, Pettersson applied to the board for an additional fellowship, this time for his own expenses in Vienna. As he argued, the grant "would enable me not only to pursue my own investigations but also to go on training and supervising a number of younger collaborators who were engaged in important problems related with artificial disintegration."75
48It was at this same point that Meyer stressed in his own letter of recommendation to the International Education Board that "His [Pettersson's] impulsiveness acts most beneficially on his young collaborators here and he knows excellent how to introduce them to the methods of this difficult subject, so that, also in this respect the object of the Educational Board, that is the higher training of young workers, would be served."76
49Pettersson was slightly over the age limit set by the board but, as Trowbridge argued, "The man is thoroughly energetic with an enormous program of work in mind, an indefatigable worker, and from this point of view in every way, compares favorably to a considerably younger man." In the usual manner of the IEB's business, Trowbridge visited Pettersson in Stockholm to interview him. Pettersson had the ambition to become a professor at the University of Stockholm, succeeding Svante August Arrhenius, the Nobel laureate in chemistry in 1903. Arhenius actually owed an old debt to Hans. Otto Pettersson was the one who first emphasized the originality of Arrhenius's dissertation in 1884 and helped him to get a docentship at Uppsala in physical chemistry, the first in Sweden in this new branch of science. Besides Arrhenius's confirmation that "He is in the first line among young men who could be thought of for a professorship in physics or physical chemistry," Pettersson's appointment would largely depend on his ability to demonstrate a similar ingenuity as his predecessor. His work in Vienna was essential in increasing the probability of getting the position.77
50Recognizing the awkwardness of the occasion, Trowbridge approached Manne Siegbahne, professor of physics at the University of Uppsala and a second candidate for Arrhenius's position. Having this in the back of his mind, Trowbridge heard Siegbahne's critique of Pettersson with reservation: "brilliant but uncritical . . . all right as an investigator, thought not sufficiently critical, but could be no good as director." Siegbahne went on to propose that the IEB should choose a "good man" from among those who had already been connected with the Radium Institute to direct Pettersson's project. The one he specifically indicated was Georg von Hevesy who was then working with Niels Bohr in Copenhagen.78 However, Trowbridge's final recommendation was in favor of Pettersson's fellowship. On December 2, 1925, the board decided to award 250 dollars per month for a year—much higher than the ordinary amount of 182 dollars—to Pettersson who immediately moved back to Vienna. A similar request to the IEB to fund Kara-Michailova as his assistant through a fellowship was declined.79
51Once again in the autumn of 1926, Pettersson asked the IEB for the prolongation of his own fellowship for another year. The work that had been done in the meanwhile in Vienna and the contradictory results of the Cambridge group puzzled Trowbridge. It was obvious from the visits of other members of the IEB that there was activity and an apparent new lease on life at the institute. Pettersson's presence had definitely stimulated work there, but Rutherford's opinion was quite different. Satisfying Trowbridge's request to assess the work in Vienna, Rutherford claimed that Pettersson had certainly galvanized the institute into "at any rate, a form of life," but he and his collaborators were "experimenting in a field which is full of traps for the unwary." He continued to say that they rushed their experiments too much and ending up not contributing anything important to the field. Given their ongoing competition, Rutherford's comments were no surprise.
52Marie Curie was more generous. When Trowbridge directed similar questions to her and mentioned some criticism that had arrived at this office, Curie laughed. "One is from Rutherford," she guessed, and went on to say that she had personally verified some of the results claimed in Vienna. As Curie emphasized, Rutherford's strategy was to belittle any work in his field not performed by one of his own students. She suggested the prolongation of Pettersson's fellowship, and this seemed to have made the difference in Trowbridge's final recommendation. Pettersson was eventually reappointed by the IEB for 1926, which proved to be the most critical year of his career.80
Putting the Scintillation Counter Aside
53The obvious disadvantages of the scintillation counter slowly but steadily pushed both the Cambridge and the Vienna laboratories to alter their material culture. As Pettersson reported later, "The subjective character of all observations made by the scintillation method added to the strain on the eyes of the counters which it involves, has made it most desirable to develop novel methods of studying atomic fragments, less exerting and less subject to errors."81 As a response to the Viennese threat to undermine their authority in the field, the Cambridge team employed a Wilson cloud chamber in their research. Although invented in 1895, the Wilson chamber was not incorporated into radioactivity research programs before 1923. Clinton Chaloner argues that the Wilson chamber clearly gained its impetus from the dispute over the scintillation counting procedures between the Vienna and Cambridge groups.82
54  Rudolf Holoubek at the Radium Institute, c 1925. Rutherford's response to the use of the chamber was enthusiastic
and soon the Wilson cloud chamber rendered the paths of particles for the
Cavendish people. Pettersson did not want to miss the opportunity of using
their instrument to support his own experimental results and theoretical claims. Supported
financially by the International Education Board, Pettersson ordered a Shimizu
Wilson ray track apparatus on November 21,
1923. This was a reciprocating form of Wilson's
instrument, which enabled more photographs per second than the initial one.
Within less than a month, the Cambridge and Paul Instrument Company (later the Cambridge
Instrument Company) shipped the new apparatus to the Radium Institute in Vienna.83 A young doctoral student, Rudolf Holoubek, was assigned to study the tracks of
H-particles from aluminum, carbon, and iron using the new instrument. To
increase its validity, Pettersson also used the new instrument repeatedly at
university lectures, showing tracks from atomic fragments to curious students.84
Rudolf Holoubek at the Radium Institute, c 1925. Rutherford's response to the use of the chamber was enthusiastic
and soon the Wilson cloud chamber rendered the paths of particles for the
Cavendish people. Pettersson did not want to miss the opportunity of using
their instrument to support his own experimental results and theoretical claims. Supported
financially by the International Education Board, Pettersson ordered a Shimizu
Wilson ray track apparatus on November 21,
1923. This was a reciprocating form of Wilson's
instrument, which enabled more photographs per second than the initial one.
Within less than a month, the Cambridge and Paul Instrument Company (later the Cambridge
Instrument Company) shipped the new apparatus to the Radium Institute in Vienna.83 A young doctoral student, Rudolf Holoubek, was assigned to study the tracks of
H-particles from aluminum, carbon, and iron using the new instrument. To
increase its validity, Pettersson also used the new instrument repeatedly at
university lectures, showing tracks from atomic fragments to curious students.84
55  Georg Stetter at the Radium Institute, c 1933. A second Cavendish method was also employed by the Vienna Institute. Georg Stetter, Assistent at the second Physics Institute and collaborator with the Radium Institute, constructed a mass spectrograph, adopting the principle used by Francis William Aston in England. The experiments enabled by Stetter's instrument further established that boron, carbon, aluminum, and iron were also disintegratable.85
Georg Stetter at the Radium Institute, c 1933. A second Cavendish method was also employed by the Vienna Institute. Georg Stetter, Assistent at the second Physics Institute and collaborator with the Radium Institute, constructed a mass spectrograph, adopting the principle used by Francis William Aston in England. The experiments enabled by Stetter's instrument further established that boron, carbon, aluminum, and iron were also disintegratable.85
56  Gustav Ortner with Gerhard Kirsch at the Institute for Radium
Research, undated. A young research student, Norbert Kreidl, developed a third
technique, which was an electric counter with an amplifying set of special
construction to record corpuscular radiation. The innovation of the technique
was that the atomic fragments were made audible based on Heinrich Greinacher's
original method for producing sound in a telephone by individual particles.86 Funded by an International Education Board grant, Kreidl spent two months in 1925
at Greinacher's laboratory in Bern for training.87 In 1926, Stetter and Gustav Ortner took over the research, designing a method
of electrical amplification of ionization currents that operated a
loudspeaker. By 1928, Pettersson said the method had given excellent results
with alpha particles, but the technical difficulties in making the atomic
fragments audible were still unresolved.88
Gustav Ortner with Gerhard Kirsch at the Institute for Radium
Research, undated. A young research student, Norbert Kreidl, developed a third
technique, which was an electric counter with an amplifying set of special
construction to record corpuscular radiation. The innovation of the technique
was that the atomic fragments were made audible based on Heinrich Greinacher's
original method for producing sound in a telephone by individual particles.86 Funded by an International Education Board grant, Kreidl spent two months in 1925
at Greinacher's laboratory in Bern for training.87 In 1926, Stetter and Gustav Ortner took over the research, designing a method
of electrical amplification of ionization currents that operated a
loudspeaker. By 1928, Pettersson said the method had given excellent results
with alpha particles, but the technical difficulties in making the atomic
fragments audible were still unresolved.88
57The development of a fourth technique, photographic emulsions, was assigned to Blau. The method had already been used by S. Kinoshita and M. Reinganum in the beginning of the 1910s to identify trajectories of alpha particles through emulsions. In early 1924, Blau attempted to observe recoil protons produced by alpha particles in paraffin. With weak radioactive sources, she could observe the lower energy particles, but the accuracy of the measurement was limited. The only strong source available was polonium. To prevent darkening of the plate by gamma radiation, Blau worked with polonium prepared in highly concentrated preparations by Rona. In 1925, Blau first detected the trajectories of slow protons.89
58  Marietta Blau at the Radium Institute, c 1925. As Pettersson wrote to his sister on March 7, 1926, "By indescribable tenacity,
she [Blau] has succeeded at an almost hopeless job I suggested to her two years
ago."90 Her first attempt was to observe recoil protons produced by alpha particles in
paraffin. With weak radioactive sources, she could also observe the lower
energy particles. The only strong source available was polonium which Rona knew
how to prepare. As Blau describes, "To prevent darkening of the plate by
gamma radiation, one worked with polonium which was prepared by Dr. E. Rona in
highly concentrated preparations. After a tedious series of indefinite
experiments, it finally worked in 1926, and in the following year, the method
could be applied to the disintegration of various atoms with alpha particles."91
Marietta Blau at the Radium Institute, c 1925. As Pettersson wrote to his sister on March 7, 1926, "By indescribable tenacity,
she [Blau] has succeeded at an almost hopeless job I suggested to her two years
ago."90 Her first attempt was to observe recoil protons produced by alpha particles in
paraffin. With weak radioactive sources, she could also observe the lower
energy particles. The only strong source available was polonium which Rona knew
how to prepare. As Blau describes, "To prevent darkening of the plate by
gamma radiation, one worked with polonium which was prepared by Dr. E. Rona in
highly concentrated preparations. After a tedious series of indefinite
experiments, it finally worked in 1926, and in the following year, the method
could be applied to the disintegration of various atoms with alpha particles."91
59Besides designing new apparatus for tracing particles, the Vienna group was apparently in need of radioactive sources, preferably polonium, which was extensively used in the artificial disintegration experiments. Irene Curie was one of the few experts within the radioactivity community who could extract and prepare polonium sources. The process involved the tiresome task of the chemical separation of the element, its purification, and the final concentration on a small surface.92 Since polonium did not emit beta particles that usually interfered in scintillation counting, its use as a radioactive source was most advantageous. Used mainly in the Wilson chamber experiments, the Viennese group was anxious to obtain the technical expertise for preparing polonium sources.93
60In May 1926, Pettersson reported to his father. "I have now managed to get Meyer to write a letter to Curie asking to send one of our scientists, Frau Doctor Rona, chemist and specialist in polonium, to her lab for three weeks in order to learn the art from Irene Curie . . . If she is allowed to go, we have no problems next year and can make our own polonium samples."94 Probably drawn from his own problems with the Curies, Pettersson added, "I first had an idea of going myself but desisted for the reason that a man coming to the Paris lab will be getting a much less friendly welcome than a woman."95 His father, most likely using his authoritative way, had already tried to persuade Curie to accept his son in her lab just a few years earlier, but she refused.96 Nevertheless, Curie accepted Rona when, not surprisingly, Pettersson succeeded in obtaining a small grant from a Swedish sponsor to fund her trip.97
61With the credential of working with some of the most important members of the radioactivity community, Rona had entered the Radium Institute during the academic year 1924–25.98 Adopting the methods of Pettersson's group on February 10, 1926, she presented to the Austrian Academy of Sciences her work on improved methods for measuring the absorption and range of H-rays and the use of polonium as a more suitable source than radium C. The main instrument she used was the scintillation counter. She teamed up with Blau and worked on the ionization of H-rays.99 Four months later, after Pettersson's persistent attempts, Rona arrived at Curie's institute to get trained in preparing polonium sources.100 On Rona's return to the Radium Institute a few weeks later, Curie was generous enough to donate to the Viennese a strong polonium source concentrated on a small silver disc of 12 mm2. Most of the following studies on artificial disintegration performed at the institute were done using either Curie's source or Rona's preparations.101
The Scintillation Counter as a Cultural Hybrid
62It was in the midst of the busiest times at the Radium Institute with Pettersson's group working feverishly on new, more reliable techniques for counting particles produced by atomic disintegrations that Kara-Michailova went further into the design and construction of the scintillation counter. On May 5, 1927, she presented her next scintillation study to the Vienna Academy.102 The focus of the study was on the brightness of scintillations produced by H-particles in relation to their velocity. As she pointed out, the most important question for the application of the scintillation method was to determine the lower limit of particles' velocity at which the scintillations were noticeable to the observer. Kara-Michailova's steps in designing her new experiment involved a noteworthy exchange of instrument parts with Stetter's mass spectrograph.
63Although in search of other methods, Stetter and his colleagues were still faithful to the scintillation counter. By modifying Aston's mass spectrograph, Stetter was first to replace the photographic plates with Pettersson's and Kara-Michailova's model of the scintillation counter.103 Purchased in 1926, Stetter's new apparatus was far too costly at close to 140 dollars. By comparison, Kara-Michailova's entire annual salary for the same year was a bit more than double that.104 Thus, replacing the photographic plates with the old, familiar scintillation screens and a microscope was not a matter of cutting down in expenses. Rather, it was an expression of loyalty to the material culture of the institute. Most of all, it revealed the commitment of the Viennese group to an experimental tradition that trusted the coworker and his or her report instead of the static, visual representation of phenomena on photographic film.
64The transfer of instrument parts, however, went both ways. "I am obliged to offer many thanks to Herr Dr. Stetter," Kara-Michailova acknowledged in her paper, "for letting me use his apparatus as well as for his help with the research."105 Literally from her working bench to Stetter's and back again, the transformation of the instrument was indicative of a dying experimental culture. Based on the fragile eyesight of the observer, the scintillation counter was put aside as experimenters sought for more trustworthy and objective methods of research.106 The replacement of human observers by mechanical devices indicated a shift in the material culture of the laboratory. Struggling to survive the transformation, the Viennese experimenters constructed instruments as hybrids at the intersection of the two material cultures.
65 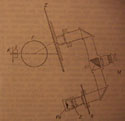 The scintillation counter used by Elisabeth
Kara-Michailova in 1927. By working in
Pettersson's group, Kara-Michailova shared a strong network of collaborators in
preparing radioactive sources such as polonium (Po), constructing scintillation
screens (Z), and measuring the absorption of gray glass (G) used in determining
the ratio in the brightness of the scintillations. With the comparison
microscope, Kara-Michailova was able to observe the scintillations of both
alpha and H-particles as she was used to in her previous experimental settings.
The new feature was the magnetic field (F) which separated H-particles
according to their velocity. Therefore, only rays of the same velocity fell
upon the scintillation screen (Z). (K) was the radioactive source and (S) was the
gap through which the rays were directed toward the magnet. This part of the
device was enclosed in a vacuum.
The scintillation counter used by Elisabeth
Kara-Michailova in 1927. By working in
Pettersson's group, Kara-Michailova shared a strong network of collaborators in
preparing radioactive sources such as polonium (Po), constructing scintillation
screens (Z), and measuring the absorption of gray glass (G) used in determining
the ratio in the brightness of the scintillations. With the comparison
microscope, Kara-Michailova was able to observe the scintillations of both
alpha and H-particles as she was used to in her previous experimental settings.
The new feature was the magnetic field (F) which separated H-particles
according to their velocity. Therefore, only rays of the same velocity fell
upon the scintillation screen (Z). (K) was the radioactive source and (S) was the
gap through which the rays were directed toward the magnet. This part of the
device was enclosed in a vacuum.
66During the preparation and the performance of her experiments, Kara-Michailova acted as the experienced and mature researcher on scintillation counters, having total control over her instruments. She employed two groups of trained observers in order to report simultaneously the scintillations produced by alpha and H-particles. The microscope in use had two Watson objectives of numerical aperture 0.70 and a single eyepiece. According to Kara-Michailova's results, the brightness of the H-particles was proportional to their residual range. The lower velocity limit for observing them was found to be less than 109 cm/sec.
67While Kara-Michailova sought to improve the scintillation measurements, Pettersson visited Cambridge in May 1927. His long discussions with Blackett and Chadwick were centered on the scintillation method. On May 16, the discussion opened up with a reference to the properties of scintillation substances and the fatigue of the observers. As Pettersson argued, his colleagues in Vienna counted for 30 seconds or often for 20 seconds instead of one minute as the Cambridge observers did.107 Both Pettersson and Chadwick placed an enormous importance on what essentially was Kara-Michailova's project, the possibility of distinguishing between flashes of alpha and H-particles using the scintillation counter. As it turned out, the resolution of the entire controversy was based on this distinction. After a "somewhat heated discussion" with Rutherford, Pettersson was finally satisfied that the British considered the Viennese way of working "worth looking into."108 In the hope of arriving to a conclusive settlement, Chadwick planned a visit to Vienna in December 1927. Up to that point, Kara-Michailova, Blau, and Rona had come a long way in designing their instruments and their experiments. Their role in the wake of Pettersson's research group was indeed instrumental.
James Chadwick's Visit to Vienna
68After Pettersson's visit to Cambridge in May 1927, things seemed to settle down and the relations between the two laboratories greatly improved. As director of the institute, Meyer took the initiative to thank Rutherford for the warm hospitality with which he accepted Pettersson in Cambridge. In an attempt to clear up any misunderstandings, he assured Rutherford that what was at issue was certainly not his authority in the field. "We hope that you got the impression that Dr. Pettersson and his coworkers here are doing their best to improve the knowledge founded by you on the atomic disintegration and that they are trying to further the work in the most serious attempts and not to trouble the advancements."109 Chadwick's visit had already been arranged for December that same year.
69That summer, however, and before Chadwick's arrival in Vienna, Bates unexpectedly visited the institute. The research had slowed and most of the personnel were absent, including Meyer who was spending his holidays at his summer residence in Bad Ischl, upper Austria. Pettersson had just returned from Sweden. "Yesterday, I was surprised with a visit from a gentleman with a lady," he reported later to Meyer. "That was our old enemy Dr. L. F. Bates who I had shortly met in London." As a genial host, Pettersson gave him and his wife a tour at the institute and they discussed in a "friendly tone." It was so friendly that Bates mentioned to Pettersson their Cambridge stock in capillary spent radon tubes from hospitals used in the preparation of polonium. Pettersson did not miss the opportunity. He immediately prompted Meyer to ask Rutherford for a supply of small radon tubes. In spite of their conflicts, they would have more than welcomed the radium—a valuable trafficking material—in the poorly supplied Vienna institute.110
70Hoping to resolve the disagreements between the two laboratories, Chadwick arrived in Vienna on December 7, 1927. A scrutiny of the scintillation screens and the most basic aspects of the artificial disintegration experiments persuaded Chadwick that the problem resided in the Viennese protocol of scintillation counting.111 As Hughes interprets Chadwick's thoughts, "There only remained the possibility that the Viennese were mistaken in believing that they could distinguish between scintillations due to alpha particles and those due to H-particles by the difference in brightness, a practice which the Viennese vehemently defended."112 It was Kara-Michailova who "vehemently" defended her method in front of the scintillation counter and during the replication of the experiments. She was among the "young ones" who stood around "stiff legged and with bristling hair," as Chadwick vividly described it.113
71Friday, December 9, "ended up with a fierce and very loud discussion."114 Apparently, Chadwick interfered on Pettersson's territory and questioned his authority in his own laboratory. As he reported to Rutherford, "It is essential that I should prepare the experiment. So far, I cannot get Pettersson to agree to this."115 Chadwick had probably suppressed the fact that when Pettersson visited Cambridge a few months earlier, he treated him as a tourist. As Hughes put it, Pettersson indeed remained a "disengaged witness" in his antagonist's territory.116 Similarly, during the first two days of his visit, Chadwick was tenaciously kept away from the scintillation screens and the microscopes. On Monday December 12, however, Chadwick took advantage of Pettersson's absence and, treating the personnel as inexperienced students, he assumed control at the institute.117
Today I arranged that the girls should count and that I should determine the order of counts. I made no change whatever in the apparatus, but I ran them up and down the scale like a cat on a piano—but no more drastically than I would in our own experiments if I suspected any bias. The result was that there was no evidence of H-particles.118
72Chadwick's bias probably did not concern the experimental techniques, but instead the female experimenters. Here is how he described to Rutherford the testing of Schmidt's apparatus for the disintegration of carbon by polonium A-particles: "Their counters, two girls, managed to find a few [scintillations]."119 The shift from "experienced observers," in Rutherford's language, describing scintillation counting in his lab, to "girl counters," in Chadwick's report of the work done in Vienna is more than a naïve linguistic slip in reference to the same task. 120 Instead, it indicates the gendering of skills.
73The counting of scintillations, so crucial for the early experiments on artificial disintegration, was treated as a highly skilled task of experimental observations when performed by Rutherford's male team in the Cavendish Laboratory. Chadwick considered the same task, performed in the Viennese setting by the female experimenters, to be disconnected from observation and its meaning to the experimental process. As he noticed, "Not one of the men does any counting. It is all done by three women."121 The immediate inference was that those women were mere counters. Nonetheless, as he described to Rutherford, he was the one who arranged that "the girls should count."
74Apparently, the three women were Kara-Michailova, Blau, and Rona.122 All of them were active participants in the controversy. Besides Kara-Michailova's work on the scintillation method, Rona was the polonium expert in the institute and the one who introduced Schmidt to the technique of preparing polonium sources while they worked on the penetration of polonium to metals. Further, in collaboration with Blau, Rona employed polonium to the study of ionization of H-rays. Blau had been working on the alternative method of photographic emulsions. What Chadwick saw, nevertheless, was routine counting and, as he later acknowledged, "the young women were perfectly honest."123
75Chadwick's presupposition, though, legitimized the way historians of science Peter Achinstein and Owen Hannaway interpreted the episode in 1985. Chadwick suspected that women were informed about the experiments in advance and "they were seeing what they expected to see."124 The minds that designed the experiments were, in Chadwick's account, separate from the eyes that recorded the results. Women's skills were limited to counting, recording, and following orders from their male colleagues.
76If one asks whether Chadwick's version of the work in Vienna was entirely his interpretation, the answer is no. Pettersson seems to have played a role in this wrong account. According to Chadwick, "Pettersson says the men get too bored with routine work [scintillation counting] and finally cannot see anything, while women can go forever." According to Chadwick's later recollections, Pettersson also argued that women were more reliable than men as scintillation counters because they could not be thinking while observing. He specifically preferred women of "Slavic descent" as counters, believing that they had superior eyesight.125 In the intense environment of the replication of the experiments, it is plausible that Chadwick and Pettersson misunderstood each other. A stiff Englishman "completely lacking a sense of humor," Chadwick had difficulties in understanding the humor of a Bohemian Swede.126 Besides, Pettersson had used a similar argument concerning the vision of the scintillation observers for describing the work of R. L. Hasche, the young American physicist from John Hopkins University who visited Vienna in 1926. His task was to compare the different kind of microscopes used in Cambridge and Vienna for counting scintillations. "Owing to his particularly good eyes," as Pettersson stressed, "he seems to be especially qualified for that kind of work."127
77It is also hard to reconcile Chadwick's claims either with Pettersson's anti-hierarchical style as the research leader of his group or with the respect he paid to his colleagues. Writing his report to the International Board of Education in 1928, Pettersson used language that surprises the reader even today. "Each collaborator has his or her particular share to take in making the practical preparations necessary for an experiment. Besides each has his or her particular theme for research which he pursues and where he can count on the help from one or more of his fellow workers." Appraising his experience of working in Vienna, Pettersson continues, "I have learned the very high value of teamwork for attacking the intricate problems that are found within the comparatively new field of nuclear physics."128
78In this collegial atmosphere, Kara-Michailova had already adopted the research on scintillation counters as her main project contributing productively to Pettersson's team. As I have already argued, Kara-Michailova was actually the one who, in collaboration with Pettersson, had altered the optical system to suit the needs of the experiment. Additionally, she offered her expertise in fluorescence and lighting measurements in order to improve the method and distinguish between alpha and H-particles, an essential issue in the Cambridge-Vienna controversy. She even introduced trained observers to her experiments, proving the seniority of her position within the team.129 As an experienced experimenter, she had absolute control of her instrument, knowing its tricks better than any of her male colleagues. Clearly, she did a great deal more than to count scintillations in front of a microscope.
79Apparently, scintillation screens were easily prepared at the institute, the procedure proving as easy as spreading zinc sulfide over a glass screen and brushing it out very smoothly. Kara-Michailova was also skilled at experimenting with different scintillating substances and preparation methods. The manipulation of the numerical aperture and the field of view of the microscope were also within her capacity. Obviously, she had control over every part of the scintillation apparatus and was able to take the entire tabletop instrument apart and reconstruct it from scratch. Counting the scintillations was not a secondary, routine task for Blau, Rona, or Kara-Michailova. On the contrary, what Chadwick failed to recognize was that all these tasks demanded individual attention and defined part of the team's identity as experimenters in the institute. It appears that Chadwick was too absorbed by his own struggle supporting the Cavendish experimental results to understand the teamwork involved.
80Focusing on laboratory technologies and gender in the two laboratories, it becomes apparent that there is a shift in the epistemological meaning of the concept of skill from an objectively quantifiable quality to an ideological category assigned to men and women on the grounds of gender biases. As the study of the scintillation counter reveals, the definition of a position as "skilled" depends on the gender identity of the person performing it. Therefore, skill becomes a political concept in the laboratory and plays a central role in maintaining the gender division of labor.130
81Scientists and even historians of science are more inclined to see women as users than as designers of the technologies they encounter in their everyday laboratory practice. For example, through the eyes of the sociologist of science Jan Golinski, the women in Vienna who participated in the dispute between the two laboratories were the "support staff" that "bore the responsibility for worrying about discrepancies in observations." As he argues, "this was found to be more of an agreeable solution than blaming the physicists on either side, presumably at least in part because the technicians were not consulted and their judgment could more easily be doubted."131 Golinski echoes Achinstein's and Hannaway's argument that, in the Radium Institute, "a division of labor had separated the eyes of the observers from the minds of the experimenters."132 All of the women who were part of the lengthy exchange of publications in the 1920s, however, studied physics, had a background in mathematics, and had experience in experimental physics.
82Kara-Michailova's effort was to secure a method that, although highly important in the 1910s and the beginning of the 1920s, was at its end as radioactivity was rapidly heading toward nuclear physics. An essential member of her research group, she had a "volcanic energy" at the peak of the controversy, as Pettersson admitted.133 Yet passion for scientific work was not enough. With the total monthly budget of 250 dollars coming from the Rockefeller grant, Pettersson was trying to manage his entire research team.134 It was therefore worth trying to save the cheap scintillation counter. A microscope with a couple of lenses, scintillation screens, and absorbing foils, was priced around 86 dollars, while a Wilson chamber lamp alone cost 14 dollars.135 Low cost was not the only advantage of the scintillation counter. For Kara-Michailova and the rest of Pettersson's team, to save the method meant to retain control over the material culture of their laboratory. For instance, as a young research student, Karlik was one of those who focused on saving the instrument.
83Two months before Chadwick visited the Vienna Institute in 1927, Karlik defended her thesis to Meyer and Hans Thirring.136 Not surprisingly given the importance of the scintillation method, Karlik's topic was the dependence of scintillations on the condition of zinc sulfide and the nature of the scintillation process.137 She described a photometric method for determining the relation between the range of alpha-particles and the brightness of the scintillations for differently prepared zinc sulfide screens. In her effort to defend the scintillation method, Karlik introduced photographic plates in her model of the counter. In order to reduce the light entering the eye through the microscope, she placed photographic plates between the objective and the eyepiece.
84Especially innovative, the Viennese group was characterized by their attempts to save an old technique by taking advantage of the elements and instrument parts of new ones. Their colleagues in Cambridge were clearly shifting from the old, shaky laboratory technologies that the scintillation counter represented to the new, persuasive evidence of the photographic plates. Instead, the Viennese were hesitantly swinging between the two worlds, transferring pieces of instruments from one workbench to another and persistently defending the old physics experimental tradition to which the scintillation counter belonged. The portable, tabletop scintillation counter which almost all of the Viennese experimenters in Pettersson's group could control and construct offered them a feeling of security, not only in the fast moving world of radioactivity research, but also in the politically unstable Viennese setting.
The Politics of a "Private" Resolution
85When Chadwick met Meyer in the latter's office at the Radium Institute on December 14, 1927, to discuss the awkward outcome of his visit in Vienna, there was anxiety on both sites. Chadwick had demonstrated that the Viennese researchers had been performing unreliable experiments using the scintillation technique. Meyer offered, on the other hand, a public acknowledgment of Chadwick's results. In this odd situation, Chadwick counter offered a private resolution following Rutherford's earlier wish to "better discuss these divergences of view in private than in print."138 At issue were not only the fame of the Vienna Institute and the authority of the Cambridge lab. Equally at stake was the reliability of the scintillation counter.
86For at least the next two years, the scientific community remained largely unaware of the outcome of the Cambridge-Vienna controversy.139 Although the scientific credibility of the Radium Institute and its researchers were not widely affected given the private resolution of the episode, Pettersson's team collapsed financially. The Radium Institute was already in a bad shape. Trowbridge had made it clear to Meyer even as early as June 1927 that without any additional local money, they could not renew their grant. Both Rose and Trowbridge were of the opinion that the board should "oppose emergency help being continued so that it would not become a crutch on which an institution leans."140 Meyer, they suggested should definitely seek support from local and other sources. Taking the suggestion seriously, Meyer first addressed the Austrian Ministry of Education which promised to support the institute with 3,500 schillings and the salary for another scientific assistant, all of which amounted to almost 1,000 dollars per year. Also, a month before Chadwick came to Vienna, Meyer raised the issue of the radium that the Austrian Academy of Sciences had lent Rutherford before the war, hoping to guarantee a yearly contribution by the investment of the expected payment to another 1,000 dollars for around 15 years.141 He wrote to Rutherford:
Austria is so impoverished, that neither the government nor private persons or societies here are in the position to keep our institute going without financial help from abroad. For the last three years, we were assisted by the International Education Board; but as the board no longer wishes to continue the appropriations to us, our funds are threatening to run down so that we must try to get money from somewhere else if this institute is to be able to go on working. So I take the liberty of asking you if you could manage to raise the necessary funds for buying the rest of the radium that you have in Cambridge perhaps in ten or fifteen years installments? The market price of radium is at present 70 dollars for 1 mg, but I am sure that our academy would be willing to accept a lower price.142
87In his response, Rutherford said he was willing to discuss the matter with the university authorities. He argued, though, that the amount of radium was only 250 mg and not the 304 mg that Meyer proposed.143 Excerpts of old notes from Rutherford's "radium book" provided evidence for his claim. By offering his own data, Meyer questioned Rutherford's estimations, but since the institute was in desperate need of financial help, the former concluded, "As there are too many uncertainties and to avoid all difficulties, I immediately propose to accept your estimation of 251 mg as the basis of all further negotiations."144
88While Chadwick was still in Vienna, Meyer had the chance to discuss the issue thoroughly with him. On December 21, 1927, immediately after Chadwick's return to Cambridge, Rutherford settled the matter. "I am very sensible of the generosity shown by the Vienna Academy of Sciences and the Austrian government in loaning me such a valuable preparation for such a long period . . . Please let me know if the price of purchase (3000 pounds) and the mode of payment by installments is satisfactory to you."145
89Eventually, as Meyer succeeded in raising funds from local and other international sources, Trowbridge recommended a grant of 3,000 dollars for the year 1928–29 and 1,500 for the year 1929–30 which was actually the last payment from the Rockefeller Foundation.146 "I really think," Trowbridge bragged to Rose, "we can be self-congratulatory on the outcome of this financially minor but scientifically fairly major undertaking on our part. I think this case of the Radium Institute forms a good argument for continuing and extending assistance to centers not of general major interest, but in which unique good elements may be found." What he saw as a "somewhat moribund institute" in the early 1920s, remained a peripheral center in Trowbridge's mind until the board's assistance came to an end in 1930, strongly defining the institute's scientific fate.
90While the board renewed the grant to the Radium Institute for two more years, Pettersson was not as lucky. His fellowship came to an end after Chadwick's visit to Vienna. In a letter to his sister in 1928, Pettersson described his difficult life in Vienna:
My colleagues have been touching at looking for somewhere to live for us. The Pension Atlanta would have been too costly. We are now renting two rooms in the flat of an elderly lady in Döbling, a quiet, country-like suburb of Vienna. With time, we have been able to worm ourselves into the lady's confidence, even using her kitchen for making lunches. For dinners, we have found a good, simple Gasthaus at the Silbergasse. Dinner for three amounts to less than 5 shillings (2.5 sek) so, the affluent times of Rockefeller are gone. Kara (Michailova) who helped much in getting us rooms, also has put in quite a supply of butter, marmalade, bread, eggs, and oranges, welcoming us at our arrival on Good Friday. Gerhard Kirsch's sister had put flowers in both rooms and from Blau and Rona, there were Easter eggs and a set of Dominoes for Anne.147
91Unable to continue his research without the Rockefeller grant, Pettersson eventually returned to Sweden. According to Stuewer, "The entire investment collapsed to the ground in a few short days in December 1927. It was a severe shock."148 The tragic picture that Stuewer draws does not do justice to the collaboration that followed Chadwick's visit to Pettersson and his colleagues in Vienna. It was not until 1936 that as Karlik wrote to him: "To think that this has been your last regular visit regarding our collaboration in atomic physics is very sad. I can hardly realize it yet. This joint work has been so much the center of my interests for the past years that it will mean quite a readjustment of my inner life to get adapted to the new circumstances." Acknowledging Pettersson's role in her career, Karlik added, "It's you who have shown me what experimental physics really is; if I look back, I must say I had only a very faint idea of it before I began working with you."149
92Chadwick’s visit, though, led Pettersson to a different kind of tragic circumstances other than the immediate collapse of his work with the Viennese. The defeat for Pettersson was personal since he lost the battle over his father's wishes. In 1928, he was refused a professorship of physics at the University of Stockholm. Instead, his father Otto arranged money for a new professorship, this time on oceanography in Göteborg.150 Pettersson never gave up his interest in radioactivity research, but he also never acquired a position in physics. After years of attempts to avoid his father's influence, Pettersson had to rely on him once more for his academic career. It was not until 1934 that he admitted to Karlik, "I am of course aware that I am counted out before the physicists of Europe, 'suspect d'otre suspect,' a position I do not mind so much personally, but which is of course unfortunate for the work."151
93The Cambridge group, financially and scientifically, remained on the top of the game. Early in 1928, Rutherford was aware of the importance of wave mechanics to radioactivity and succeeded in getting a new position for a teacher in theoretical physics and additional money to organize a conference on beta and gamma rays.152 As Andrew Brown argues, "The main purpose of calling the conference was for Rutherford, Chadwick, and Ellis to make sure they had not left anything important out of their forthcoming book."153 The conference, nevertheless, was also a forum where the Cambridge team planned to restore their authority in the field of radioactivity after the lengthy exchange of papers in the scientific press with the Viennese. Moreover, they intended to do so undisturbed by their main opponents.
94Although they invited all the key figures of the radioactivity community, it was not by chance that only one participant from the Radium Institute in Vienna attended the meeting. Ewald Schmidt, even though he was not the one to work on the central theme of the conference, was the one to receive an invitation.154 Other young researchers who had worked on radioactivity in general attended the conference, yet the young Viennese who played an essential role in the controversy such as Kara-Michailova, Karlik, and Blau, or the men of the group such as Kirsch, Holoubek, and Ortner were silently excluded. The only other person that Chadwick thought of inviting was Stetter, but, as he mentioned to Meyer, "Our funds were not sufficient."155
95Blau especially would have been a perfect fit. She had completed a dissertation on the absorption of gamma rays and she already had a long list of publications and experience in the alternative method for detecting radiation, the photographic emulsions.156 Thus, what the Cambridge group avoided through a public acknowledgment of the episode, they better accomplished through the politics of exclusion. It was not the credibility of the researchers that was affected but rather the possibility of their presence in the scientific community.
96Suspecting this early on, Pettersson tried to prevent it in vain. As a gesture of good will, he sent Rutherford a spinthiroscope of his design for showing the hydrogen particles from paraffin. The gift was intended to temper the anxiety and tension that surrounded Chadwick's visit and ensure that Pettersson's powerful antagonists would not affect his own and his colleagues' presence in the radioactivity community. In his thankful response, Rutherford added "There are so few workers in this difficult subject that we must try and pull together and settle our differences as far as possible by private correspondence rather than by controversies in scientific journals, which in my experience do nothing but cause irritation. If you and your friends are of the same opinion, I think there should be no great difficulty in settling our differences. During my whole scientific career I have not had any serious controversy and always advise my students to be considerate where differences of opinion are involved."157 Even though the dispute was settled in private, the group at the Radium Institute was finally dissolved. Partly because of the silent exclusions that the Cambridge team imposed on them and partly because of financial difficulties, most of the members were soon scattered to other laboratories, abandoning their teamwork on artificial disintegration.
The Last Attempt to Save the Scintillation Counter
97In May 1928, Karlik teamed up with Kara-Michailova in a last attempt to save the scintillation counter. At a meeting in the Vienna Academy of Sciences, they presented their coauthored paper on the luminescence caused by alpha particles and its relation to their energy. For the first time, the luminescence produced by alpha particles emitted from polonium was measured by means of the photoelectric current of a rubidium cell. Their next coauthored paper appeared the same year in the prestigious Zeitschrift für Physik.158 Besides discussing the experimental details of the relation between the brightness of the scintillation and the energy given up from the alpha particles of the source, they suggested a theoretical hypothesis in explaining the mechanism of the scintillation process. They were concerned with the manipulation of the instrument, preparing and gauging the scintillation screens, and experimenting with several different elements. They went one step further, suggesting that the zinc sulfide possesses distinct points that are already in an active condition before they are struck by the particles. Theory and experiment came together in this study of the scintillation counter.
98At the end of 1928, Kara-Michailova was promoted to the position of Wissenschaftliche Hilfscraft at the institute.159 She was obviously the mature partner in her collaboration to Karlik, although the latter's work came to the center of attention of the Cambridge group in November 1928. Julius Chariton and C. Lea, two of Rutherford's research students, raised objections to Karlik's dissertation project, published a year earlier. As they argued, there was a considerable difference between the results obtained by Karlik's and their experiments concerning the question of how the amount of light, entering the eye from an individual scintillation, affects the number of total scintillations observed. The discrepancies were probably due to "the device used for reducing the fraction of the light from a scintillation which entered the eye."160
99Chadwick communicated the paper to the Royal Society in November 1928, but not only Karlik's results were at stake. During his visit in December 1927, Chadwick was able to show empirically that the Viennese were wrong in the number of scintillations they were claiming to count. Chariton and Lea offered a theoretical explanation of the mistake. The Viennese were actually testing the role of the numerical aperture and the magnification of the microscope designed and used by the Viennese group. It became obvious that the scintillation counter was in its last days.161 Rona described Chadwick during his visit to Vienna in 1927 as "probably just as uncomfortable in the role of the judge as we were in that of the judged."162 In fact it was both the scintillation technique and the skills of the women experimenters working in Pettersson's group that were under judgment.
100Karlik and Kara-Michailova insisted on saving the scintillation counter and presented their last coauthored paper in July 1929 to the Vienna Academy.163 This time, the focus was on the brightness of the scintillations produced by H-particles. Besides Karlik's two subsequent articles in 1930 related to the scintillation technique, none of the Viennese physicists followed up on scintillation counter research, and hardly any publication on it appeared in the Mitteilungen of the institute after 1930.164 Eventually, both women who played a central role in improving the counter abandoned the technique as the center of their research focus. Kara-Michailova teamed up with Blau and worked on the penetrating radiation of polonium.165 She retained the position of scientific assistant until March 1933, when she applied to the Austrian Federation of University Women for a Yarrow Scientific Research fellowship.
101Stefan Meyer willingly provided her a reference letter, stating that Elisabeth had been an "independent and stimulated researcher. She has decisively cooperated especially in works on disintegration, on problems of luminescence, and also on the amount of time radium emanations remain in the human body, without her name always appearing at the publications." Soon after, Kara-Michailova was awarded the fellowship and starting in 1935, she spent four years at the famous Girton Women's College.166 She was employed by the Cavendish Laboratory which was then directed by Rutherford. When he passed away in 1937, he was succeeded by W. L. Bragg. In 1939, Kara-Michailova returned to Bulgaria as an associate professor in experimental atomic physics and radioactivity at the University of Sofia. As her later publication record indicates, she crossed once more from physics to medicine, carrying over her expertise in instrument making. Furthermore, she tried to transfer the collegial atmosphere of the Vienna Institute to her new department by holding weekly tea gatherings with her students and doctoral candidates.167
102A fellowship from the International Federation of University Women allowed Karlik to spend some time away from the Radium Institute. During the academic year 1930–31, she moved to William Bragg's laboratory in London.168 Her research interests were centered on crystallography and the use of x-rays in the study of the structure of crystals. It was her knowledge of radiophysics that Karlik brought to Bragg's laboratory, working with the crystallographers Ellie Knaggs and Helen Gilchrist.169 The scintillation counter was in the past for both Karlik and Kara-Michailova, as it was for the research groups in Cambridge and Vienna.
What It Meant to Be an Experimenter at Vienna's Radium Institute
103What it meant to be a physicist specializing in radioactivity strongly depended on the culture within which such an identity was constructed and performed. In Vienna, Pettersson initiated a new era of experimentation and transformed the meaning of "experimenter" within the institute. Introducing the research program on artificial disintegration required changes in space arrangements, the use of new experimental techniques, and the reordering of the entire laboratory. Before his arrival, physicists worked either alone or with one collaborator who was often a practicum student. In contrast, in the early 1920s, the shift to experiments on artificial disintegration investigating several elements demanded group research. It was both the competition with the Cavendish Laboratory and the nature of the experiments themselves that necessitated the changes. "I have learnt the very high value of team work," admitted Pettersson about his research in Vienna in his report to the International Education Board in 1928 and he continued:
Our particular kind of work requires the close and continued collaboration of at least half a dozen highly specialized workers: one for preparing and calibrating the screens and the absorption foils used in scintillation counts; one for preparing the disintegration apparatus itself, the substances which are to be investigated in it and the gas with which it is filled; one for working the mass spectrograph and its auxiliary instruments; and one specialist on photography . . . Finally, several of the persons mentioned must act as counters in scintillation observations when the ordinary disintegration apparatus or the mass spectrographs are used.170
104To determine the number of protons produced by the disintegration of light nuclei, the physicists counted the number of flashes that appeared on the zinc sulfide screens of their scintillation counters. The task was painstaking, and several students were usually recruited to do the counting in addition to the main experimenters themselves. For instance, in her later experiments, Kara-Michailova had employed two groups of observers who simultaneously recorded the scintillations produced by alpha and H-particles.171 In all cases, these observers were characterized as "highly specialized."
105At the same time, in order to compete with Rutherford's team, each of the Viennese researchers focused on one of the key aspects of the controversy, collaborating with other colleagues to develop and improve new experimental methods or to probe a variety of different elements. Thus, Pettersson introduced research among a cluster of scientists with varying areas of expertise, each of whom was assigned a topic of investigation that contributed to the overall effort. Although Pettersson was the leading experimenter, he encouraged teamwork and exercised minimal control over the pace and direction of research in his group. He describes its rituals in the following way: "The papers are circulated in manuscripts and read by all the coworkers and thoroughly criticized them before publication."172 The Viennese chose peer review as a way to construe experimentation in their local context. The result was the development of close collaborations, the exchange of ideas and scientific papers among the researchers, and even the transfer of materials and of parts of instruments from one workbench to the other. For example, Karlik reported to Pettersson, "In the afternoon of that same Wednesday, Ortner quite unexpectedly presented me with 300 milliCuries of radium (something had gone wrong with their apparatus)."173
106In contrast, to be a physicist in the British group required accepting the hierarchical structure that Rutherford's authority imposed and working on assigned projects that were designed to maintain that authority. He was formally in charge of all research students whether they were doing experimental or theoretical work and every decision went through him. Students' training was strictly organized around the accepted research methods of the laboratory and maintained the local experimental culture. As Jeff Hughes explains, "Rutherford introduced a compulsory new training regime for fledgling experimentalists based on the course established at Manchester by Walter Makower and Hans Geiger."174 Rutherford's reprimand of his student Bates gives us a glimpse of the highly competitive environment at the Cavendish Laboratory. As director of the laboratory, he left little doubt as to the obligations of his colleagues and students. In Mark Oliphant's words:
With Rutherford looking over our shoulders, impatiently awaiting the outcome of the observation, the operator tended to make silly mistakes . . . . Once at the end of a particular heavy day, when the experiments had gone well, we decided to postpone development till the next morning when we were fresh and we could handle the long strip in new developer and fixer without damage. Just as we were leaving, Rutherford came in. He became extremely angry when he heard what we had decided and insisted that we develop the film at once . . . . We did our best, but the developer was almost exhausted and the fixing bath yellowed with use. In the end, he went off muttering to himself that he did not know why he was blessed with such a group of incompetent colleagues.175
107Quite the opposite, Pettersson's manners and his shift in emphasis from the individual researcher to the research group definitely improved collegiality at the Radium Institute. In that welcoming and less competitive atmosphere, women were more readily accepted. As the cases of Dagmar Pettersson, Kara-Michailova, and Rona suggest, they exercised the same type of control over their experiments, instruments, and theories as their male colleagues. It was these different social conditions in the two laboratories that also provided different ways to make, put in use, and sustain the scintillation counter.
^topNotes
Note 1: In his interview, Arthur Svansson stressed several times Pettersson's strong personality and his impulse for "big ideas, wild ideas." As Svansson claimed, "He was not afraid to have theories that were not easy to be proved" (Svansson, interview by the author, September 21, 2001, Göteborg). back
Note 2: Pettersson to his sister E. Mellbye, March 7, 1926 (in Swedish; Agnes Rodhe Papers, translated by Rodhe). back
Note 3: Rutherford, "Collisions of alpha-particles with Light Atoms" (1919), 589. back
Note 4: Hughes, The Radioactivists (1993). back
Note 5: Hughes, The Radioactivists (1993), 20. back
Note 6: Rutherford and Chadwick, "The Artificial Disintegration of Light Elements" (1921), 61. back
Note 7: Kirsch and Pettersson, "Über die Atomzentrümmerung" (1923); Kirsch and Pettersson, "Experiments on the Artificial Disintegration" (1923), 500–12; Kirsch and Pettersson, "Long-range Particles" (1923). back
Note 8: Kirsch and Pettersson, "Long-range particles" (1923), 395. back
Note 9: For considering instruments as historical documents, see Bedini, "The Hardware of History" (2001). back
Note 10: Julian Holland, "Scientific Instrument Makers in an Institutional Context," talk given at the XXI International Scientific Instrument Symposium, National Hellenic Research Foundation 2002, Athens, Greece. back
Note 11: See for example, Berg and Mol, Differences in Medicine (1998); Clarke and Fujimura, The Right Tools for the Job (1992); Rapp, Testing Women, Testing the Fetus (1999). For a review of the technology and gender issue, see Faulkner "The Technology Question in Feminism" (2001); Grint and Gill (eds.), The Gender-Technology Relation (1995); Technology and Culture (1997); Wajcman, "Reflections on Gender and Technology Studies" (2000). back
Note 12: For example, as Rossiter argued in 1980, the rise of big science and the flow of significant budgets at the end of the nineteenth century enabled women to enter scientific fields as support staff or assistants at a few research centers. The most illustrative example is the entry of women in astronomy with the main task to classify photographs of stellar spectra. See Rossiter, "Women's Work in Science" (1980), and Caroline Herzenberg's and Ruth Howes' book Their Day in the Sun: Women of the Manhattan Project pieces together fascinating information on women and the atomic bomb. Throughout the study, it becomes apparent that those who made key decisions and shaped laboratory technologies were not the women but the men who worked at the project; see Herzenberg and Howes, Their Day in the Sun (1999). back
Note 13: Gay, "Invisible Resource" (1996), 314. back
Note 14: Kirby, "The Discovery of Actinium" (1971), 300. back
Note 15: Crookes, "The Emanation of Radium" (1903), 407. back
Note 16: Levy and Willis, Radium and Other Radio-Active Elements (1904), 50. back
Note 17: Elster and Geitel, "Über die durch radioactive Emanation" (1903); Regener, "Über die Zählung der a-Teilchen" (1908). back
Note 18: Rutherford, "The Chemical Nature of the Alpha-Particles" (1963), 143. See also Rutherford and Geiger, "A Method of Counting the Number of Alpha Particles" (1908), 1–3. back
Note 19: Makower and Geiger, Practical Measurements in Radioactivity (1912). back
Note 20: Rutherford, "Collision of alpha particles with Light Atoms" (1919), 551. back
Note 21: Rutherford, "Nuclear Constitution of Atoms" (1920), 14–38. back
Note 22: Stuewer, "Artificial Disintegration" (1985), 240; see also Hughes, The Radioactivists (1993), 62. For issues of authorship in the discovery process, see Galison, Image and Logic (1997), 199–200. back
Note 23: Darwin, "The Discovery of Atomic Number" (1956) quoted in Hughes, The Radioactivists (1993), 4. back
Note 24: Rutherford, Chadwick, and Ellis, Radiations from Radioactive Substances (1930), 550. back
Note 25: Hughes, The Radioactivists (1993), 45. back
Note 26: Rutherford and Chadwick, "The Artificial Disintegration of Light Elements" (1921), 49. back
Note 27: Rutherford, "Bakerian Lecture, Nuclear Constitution of Atom" (1920), 381. back
Note 28: For a general history of the Cavendish Laboratory and different research styles, see Goldhaber, "Reminiscence" (1993); Growther, The Cavendish Laboratory (1974); Larsen, The Cavendish Laboratory (1964); Sviedrys, "The Rise of the Physical Sciences" (1970); Falconer, "J. J. Thomson and the 'Cavendish' Physics" (1989). For physics in Cambridge from a gender perspective see Gould, "Women and the Culture of University Physics" (1997). back
Note 29: This claim is based mainly on M. and G. Rayner-Canham, A Devotion to their Science (1997); Hughes, The Radioactivists (1993). back
Note 30: Heilbron, Ernest Rutherford and the Explosion of Atoms (2003), 102. back
Note 31: Gould, "Women and the Culture of University Physics" (1997), 149. back
Note 32: Heilbron, Ernest Rutherford (2003), 102; Hunt and Barker. Women at Cambridge: A Brief History (1998). back
Note 33: Although important, scintillation counting was not the only method that Pettersson and Kirsch were planning to use. As they announced in their first paper, the emanation capillaries enclosing the sources used in the scintillation counter "will be used in this institute also for studying atomic disintegration by the Wilson method." Kirsch and Pettersson, "Long-range Particles" (1923), 395. back
Note 34: Galison, Image and Logic (1997), 435. back
Note 35: Pettersson, "Zur Herstellung von Radium C" (1923), 55–57. The decision to replace the metal tubes with pure fused silica (quartz) was probably based on Stefania Maracineanu's recommendation that glass instead of metal plates should be used in order to obtain good results for the periods of radioactive substances; see Maracineanu, "Researches on the Constant of Polonium" (1923). For more on Maracineanu and her work at the Curie's laboratory, see Popescu, M. Rayner-Canham, and G. Rayner-Canham, "Stefanie Maracineanu" (1997). back
Note 36: Kirsch and Pettersson, "Long-range Particles" (1923), 395. back
Note 37: For a long time, it was considered that alpha particles emitted by a given substance have a definite range. By studying the emitted radiation of thorium in 1906, Otto Hahn discovered that alpha rays can have different ranges for the same source. In 1919, Rutherford established the presence of particles of range 9.0 cm from radium active deposit; see Bates and Rogers, "Particles of Long Range" (1924). With their experiments, Bates and Rogers observed even longer ranges emitted from radium C such as 9.3 cm, 11.1 cm and 13.2 cm; see Bates and Rogers, "Long-Range alpha-Particles" (1923). back
Note 38: Kirsch and Pettersson, "Long-Range Particles" (1923), 687. back
Note 39: Pettersson refers to Rutherford's letter in his own on 27 July 1923, AÖAW. back
Note 40: Pettersson to Rutherford, 27 July 1923, AÖAW (in English). back
Note 41: Pettersson to Rutherford, 27 July 1923, AÖAW (in English). back
Note 42: Bates and Rogers, "Particles of Long Range" (1924), 114. back
Note 43: Bates and Rogers, "Particles of Long Range" (1924), 114. back
Note 44: Bates and Rogers, "Particles of Long Range from Polonium" (1924). back
Note 45: Bates and Rogers, "Particles of Long Range from Polonium" (1924), 360. back
Note 46: For example, in their article published in the Proceedings in 1924, Bates and Rogers expressed their "best thanks to Sir Ernest Rutherford who suggested this research and who gave us many helpful suggestions during its progress." See Bates and Rogers, "Particles of Long Range" (1924), 116. back
Note 47: Pettersson, Dagmar "Über die maximale Reichweite" (1924), 149–62. back
Note 48: Rodhe, interview by the author, 22 September 2001, Göteborg. back
Note 49: Rodhe, interview by the author, 22 September 2001, Göteborg. back
Note 50: Svansson, interview by the author, September 21, 2001, Göteborg. See also Svansson, Otto Pettersson: the Oceanographer, the Chemist, the Inventor (2006) back
Note 51: Rodhe to Rentetzi, 29 October 2001. back
Note 52:Rodhe to Rentetzi, 29 October 2001. Hans Pettersson's report on the investigation regarding artificial disintegration of elements "Atomzertrümerung" carried out during the first half of 1926 in Radium Institute and the second Physics Institute of the University of Vienna, GUB. On couples in science, see Abir-Am, Pycior and Slack, Creative Couples in the Sciences (1996). back
Note 53: For details on Pettersson's method of the preparation of the radium C source, see Stuewer, "Artificial Disintegration" (1985), 248–9. back
Note 54: Pettersson, Dagmar. "Über die maximale Reichweite" (1924), 153. back
Note 55: Pettersson, Dagmar "Long Range Particles" (1924), 642. back
Note 56: Pettersson, "Long-Range Particles from Radium-Active Deposits" (1924), 641–42. back
Note 57: Rodhe to Rentetzi, October 29, 2001. During her stay in Vienna and despite the fact that she had two children, Dagmar remained part of the institute. From 1922 to 1926, she appeared in the Bericht of the institute as a collaborator while she followed Hans on his constant trips between Vienna and Göteborg from 1922 to 1928. Dagmar and Hans hired a Swedish nanny to take care of their kids in Vienna. Sometimes, they even brought their daughter to the institute. Rodhe recalled that during her early years, she used to observe the preparation of scintillation screens at the Radium Institute when her parents were busy with their scientific research. She was even taught how to brush the mixture of alcohol and zinc sulfide from the small rectangle glasses used for scintillation counting. Rodhe, interview by the author, 22 September 2001, Göteborg. back
Note 58: Rutherford and Chadwick, "On the Origins and Nature" (1924). back
Note 59: Hans to Otto Pettersson, August 22, 1927, in Otto Pettersson–Gustaf Ekman correspondence, Regional Archives, Göteborg. I would like to thank Artur Svansson for providing and translating this letter. back
Note 60: Hans Pettersson's report to the International Education Board, April 1928, GUB. It was during those years that Dagmar built warm, friendly relations, and close collaborations with the rest of the women that eventually lasted longer than her stay in Vienna. Blau, Karlik, and Rona occasionally corresponded with Dagmar even after Pettersson's death in the 1970s. They used to recall the pleasant years they spent together in Vienna during the 1920s. As director of the institute in the 1960s and 1970s, Karlik often conveyed information about their common friends and acquaintances. For example, Karlik reported the death of Georg Stetter's wife and Przibram's 88th birthday in Karlik to Dagmar Pettersson, December 18, 1966, GUB. back
Note 61: Pettersson, Dagmar. "Über die maximale Reichweite" (1924), 153. back
Note 62: Kara-Michailova and Pettersson, "Über die Messung" (1924). back
Note 63: Kirsch and Pettersson, "Long-Range Particles" (1923). back
Note 64: Kara-Michailova and Pettersson, "Über die Messung der relativen Helligkeit" (1924), 164. back
Note 65: Hans Pettersson's financial report to the International Education Board, 1928, GUB. back
Note 66: Kara-Michailova and Pettersson, "Über die Messung der relativen Helligkeit" (1924), 165. back
Note 67: Belar, "Spektrophotometrische Untersuchung" (1923); Przibram and Belar, "Die Verfärbung" (1923). back
Note 68: Kara-Michailova, "Quantitative optische Unterscheidung von alpha und H-Teilchen" (1924). back
Note 69: Hans Pettersson to Mellbye, March 7, 1926 (in Swedish, Agnes Rodhe Papers, translated by Rodhe). back
Note 70: Albert Einstein, November 24, 1923, Box 25/360, RAC. back
Note 71: Pettersson had even tried to acquire a grant from the General Electric Company, as he knew that they had already supported several German, British, and French scientists. Pettersson to Irving Langmuir, June 4, 1924; Langmuir to Wickliffe Rose, October 13, 1924, Box 25/360, RAC. back
Note 72: Trowbridge to Rose, April 2, 1925; Memorandum of conversation with Professor Stefan Meyer and Karl Przibram, Extract from Doctor Trowbridge's Log of visit to Vienna, Austria, March 26, 1925, Box 25/360, RAC. back
Note 73: See for example how powerful the Rockefeller Foundation was in the case of the health-related work that it funded in central Europe after the First World War. Weindling, "Public Health and Political Stability" (1993); Page, "The Rockefeller Foundation and Central Europe" (2002). For the case of physics under National Socialism in Germany, see Macrakis, "The Rockefeller Foundation" (1989); Siegmund-Schultze, Rockefeller and the Internationalization of Mathematics (2001). back
Note 74: W. W. Brierley to Trowbridge, July 6, 1925, Box 25/360, RAC; Hans Pettersson's report to the International Education Board, April 1928, GUB. back
Note 75: Hans Pettersson's report to the International Education Board, April 1928, GUB. back
Note 76: Meyer to Trowbridge, October 7, 1925, AÖAW (in English). back
Note 77: Trowbridge to Rose, October 14, 1925, Box 56/923, RAC. back
Note 78: Radium Institute in Vienna, G. V. Hevesy, Box 25/360, RAC; Trowbridge to Rose, October 2, 1925, Box 56/923, RAC. back
Note 79: W. W. Brierley to Otto Sylwan, December 2, 1925, Box 56/923, RAC; Pettersson to Meyer, December 4, 1925, AÖAW. Trowbridge to Rose, Memorandum of conversation, Box 25/360, RAC. back
Note 80: Trowbridge to Rose, October 5, 1925; Memorandum of conversation with Madame Curie, October 4, 1926, Box 56/923, RAC. back
Note 81: Hans Pettersson's report to the International Education Board, April 1928, GUB (in English). back
Note 82: Chaloner, "The Most Wonderful Experiment" (1997), 364. back
Note 83: The Cambridge and Paul Instrument Company to Pettersson, December 18, 1923, GUB. For more on the marketing of cloud chambers, see Chaloner, "The Most Wonderful Experiment" (1997). back
Note 84: Holoubek, "Der Nachweis" (1927); Holoubek, "Die Sichtbarmung" (1927). Hans Pettersson Report, Part II, April 1928, Box 56/923, RAC. back
Note 85: For Aston's mass spectrograph and the rest of the Cambridge techniques adopted in Vienna, see Hughes, The Radioactivists (1993). Also Pettersson, "On the Investigations" (1926), GUB. back
Note 86: Hans Pettersson's report to the International Education Board, April 1928, GUB; Kreidl, "Zur Verwendbarkeit des Geiger'schen" (1927). See also Greinacher, "Über die akustische Beobachtung" (1924). back
Note 87: Hans Pettersson's report to the International Education Board, April 1928, GUB. back
Note 88: Ortner and Stetter, "Die Hörbarmachung von H-Strahlen" (1927). See also Hughes, The Radioactivists (1993), 118. For the interference of Stetter and Ortner to Kreidl's project see Pettersson to Meyer, August 28, 1927, AÖAW. Hans Pettersson Report, Part II, April 1928, Box 56/923, RAC. back
Note 89: Blau, "Über die photographische Wirkung" (1925); Blau, "Die photographische Wirkung" (1925); Blau "Über die photographische Wirkung" (1927); Blau, "Über die photographische Wirkung" (1928); Blau, "Über photographische Intensitätsmessungen von Polonium" (1928). back
Note 90: Pettersson to Mellbye, March 7, 1926 (in Swedish, Agnes Rodhe Papers, translated by Rodhe). For a detailed account of Blau's work related to photographic emulsions, see Galison, Image and Logic (1997), 146–60. back
Note 91: Blau, curriculum vitae, Leopold Halpern Papers. back
Note 92: Rona, How it Came About (1978), 22. back
Note 93: As Pettersson reported in 1926, the use of a strong polonium source in Holoubek's experiments enabled him to take fewer photographs than Blackett did by working on the same method in Cambridge; see Pettersson, "On the Investigations" (1926), GUB. back
Note 94: Hans to Otto Pettersson, May 24, 1926 (in Swedish, Agnes Rodhe Papers, translated by Rodhe). back
Note 95: Hans to Otto Pettersson, May 24, 1926. (in Swedish, Agnes Rodhe Papers, translated by Rodhe). back
Note 96: Rodhe, interview by the author on September 22, 2001, Göteborg. Pettersson's uncomfortable relations with the Curies deteriorated when he visited their institute in Paris in 1936. Commenting on his letter that described the situation, Karlik suggested that "you must remember, too, what queer people they are. As regards Irene I don't think you should feel puzzled by anything she does. Her manners are really perfectly intolerable." Karlik to Pettersson, 9 April 1936, GUB. back
Note 97: Pettersson, "On the Investigations" (1926), GUB. back
Note 98: Almanach der Akademie der Wissenschaften (1925), 216, AÖAW. back
Note 99: Rona, "Absorptions und Reichweitenbestimmungen" (1926); Blau and Rona, "Ionisation durch H-Strahlen" (1926). back
Note 100: Rona to Meyer, June 3, 1926, AÖAW. The letter was sent from Paris. back
Note 101: Pettersson, "On the Investigations" (1926), GUB. back
Note 102: Kara-Michailova, "Helligkeit und Zällbarkeit der Scintillationen" (1927). back
Note 103: Stetter, "Die Massenbestimmung von H-Partikeln" (1925); Stetter, "Die Bestimmung des Quotienten Ladung/Masse" (1926); Stetter, "Massenbestimung" (1926); Stetter, "Die Massenbestimung" (1927); Stetter, "Die neueren Untersuchungen" (1927); See also Hughes, The Radioactivists (1993), 107. back
Note 104: Hans Pettersson's report to the International Education Board, GUB. back
Note 105: Kara-Michailova, "Helligkeit und Zählbarkeit" (1927). back
Note 106: For a historical account of the notion of objectivity, see Daston and Galison, "The Image of Objectivity" (1992). back
Note 107: "Discussion on atomic disintegration in Cambridge" May 16, 1927, GUB. back
Note 108: Pettersson to W. E. Tisdale, May 18, 1927, Box 56/923, RAC. back
Note 109: Meyer to Rutherford, May 25, 1927, AÖAW (in English). back
Note 110: Pettersson to Meyer, August 28, 1927, AÖAW; Pettersson to Meyer, August 31, 1927, AÖAW. back
Note 111: For a detailed description of Chadwick's visit, see Stuewer, "Artificial Disintegration" (1985); Hughes, The Radioactivists (1993); Brown, The Neutron and the Bomb (1997). back
Note 112: Hughes, The Radioactivists (1993), 112. back
Note 113: Hughes, The Radioactivists (1993), 134. back
Note 114: Stuewer, "Artificial Disintegration" (1985), 285. back
Note 115: Stuewer, "Artificial Disintegration" (1985), 285. back
Note 116: Hughes, The Radioactivists (1993), 136. back
Note 117: As Chadwick wrote to Rutherford, Pettersson's whole family came to visit him in Vienna; see Stuewer, "Artificial Disintegration" (1985), 287. back
Note 118: Stuewer, "Artificial Disintegration" (1985), 286. back
Note 119: Stuewer, "Artificial Disintegration" (1985), 286. back
Note 120: Rutherford, Chadwick, and Ellis, Radiations from Radioactive Substances (1930), 550. back
Note 121: Stuewer, "Artificial Disintegration," (1985), 287, excerpt from Chadwick's letter to Rutherford on 12 December 1927. back
Note 122: Pettersson to Kerr Grant, December 29, 1927, GUB. back
Note 123: Rona and Schmidt, "Untersuchungen" (1927); Blau and Rona, "Ionisation durch H-Strahlen" (1927); Blau, "Uber die photographische" (1925). Brown, The Neutron and the Bomb (1997), 88. back
Note 124: Achinstein and Hannaway, Observation, Experiment, and Hypothesis (1985); Brown, The Neutron and the Bomb (1997), 88. back
Note 125: Stuewer, "Artificial Disintegration" (1985), 287. back
Note 126:Rona, How it Came About (1978), 20. This is how Rodhe characterized her father; Rodhe, interview by the author, September 22, 2001, Göteborg. back
Note 127: Pettersson, "On the Investigations" (1926), GUB. back
Note 128: Hans Pettersson's report to the International Education Board, April 1928, GUB (emphasis added). back
Note 129: Kara-Michailova, "Helligkeit und Zählbarkeit" (1927). back
Note 130: I owe my thanks to Peter Galison for his valuable suggestions on the concept of skill. back
Note 131: Golinski, Making Natural Knowledge (1998), 90. back
Note 132: Achinstein and Hannaway, Observation, Experiment, and Hypothesis (1985), x. By working with Roger Stuewer's precise historical account of the episode, the above historians and sociologist jump to a conclusion that Stuewer never argues for. He not only reports on women's scientific work but is willing to look more carefully at Chadwick's interpretation of a derogatory comment on women's work supposedly made by Pettersson. As Stuewer argues, "Pettersson valued his coworkers highly, and they him, and hence, it is not clear in what tone of voice he made these remarks." What Stuewer did nevertheless was to write the story of the dispute between the two laboratories from the point of view of the male protagonists. Rutherford, Chadwick, Pettersson, and Kirsch did have their fair share in the historical plot and, although acknowledging some of the women, Stuewer left them on the margins of his narrative. Their absence is what permits accounts such as Golinski's, Achinstein's, and Hannaway's. back
Note 133: Pettersson to Mellbye, March 7, 1926 (in Swedish, Agnes Rodhe Papers, translated by Rodhe). back
Note 134: Pettersson to Mellbye, March 7, 1926 (in Swedish, Agnes Rodhe Papers, translated by Rodhe). back
Note 135: Hans Pettersson's financial report to the International Education Board, GUB. back
Note 136: Karlik's exams for her major in physics were on October 10, 1927, and for mathematics on January 13, 1928. She finally graduated on March 8, 1928 (Rigorosenakt 9765, AUW). back
Note 137: Karlik, "Über die Abhängigkeit der Szintillationen" (1927). back
Note 138: In a letter of July 19, 1924, Rutherford suggested to Pettersson to resolve their differences in private. "Workers in this field" as he argued "are too few and too select to misunderstand one another." See Hughes, The Radioactivists (1993), 138–9. Stuewer, "Artificial Disintegration" (1985), 256. back
Note 139: Hughes, The Radioactivists (1993), 351, 206; Brown, The Neutron and the Bomb (1997), 88; Weiner, Sir James Chadwick, oral history, AIP. back
Note 140: Memorandum, conference between Wickliffe Rose and Augustus Trowbridge, June 13, 1927, Box 25/360, RAC. back
Note 141: Meyer to Trowbridge, January 20, 1928, Box 25/360, RAC. back
Note 142: Meyer to Rutherford, November 8, 1927, AÖAW. back
Note 143: Rutherford to Meyer, November 23, 1927, AÖAW. back
Note 144: Meyer to Rutherford, November 29, 1927, AÖAW (in English). back
Note 145: Rutherford to Meyer, December 21, 1927, AÖAW. Hughes argues that Meyer agreed with Rutherford's terms not only because he was financially desperate but also because he was embarrassed by the outcome of Chadwick's visit; see Hughes, The Radioactivists (1993), 141. The letters exchanged before Chadwick's visit, nonetheless, show that Meyer had already agreed with Rutherford's terms. On the contrary, it was Rutherford who seems to have been embarrassed after Chadwick's visit to Vienna. back
Note 146: Almanach der Akademie der Wissenschaften (1930), 235, AÖAW. Trowbridge to Rose, February 7, 1928, Box 25/360, RAC. back
Note 147: Pettersson to Mellbye, April 15, 1928 (in Swedish, Agnes Rodhe papers, translated by Rodhe). As Rodhe recalled, "I can remember those dinners for two, each parent scratching morsels onto my extra plate." Rodhe to Rentetzi, October 29, 2001. back
Note 148: Stuewer, "Artificial Disintegration" (1985), 290. back
Note 149: Karlik to Pettersson, March 28, 1936, GUB (in English). back
Note 150: Rodhe, interview by the author, September 22, 2001, Göteborg; Svansson, interview by the author, September 21, 2001, Göteborg Svansson, interview by the author, September 21, 2001, Göteborg. back
Note 151: Pettersson to Karlik, September 27, 1934, GUB. back
Note 152: Hughes, The Radioactivists (1993), 132, 152. back
Note 153: Brown, The Neutron and the Bomb (1997), 96. back
Note 154: Hughes, The Radioactivists (1993), 152–157. Chadwick to Meyer, June 23, 1928, AÖAW. back
Note 155: Chadwick to Meyer, June 23, 1928, AÖAW. back
Note 156: Blau, "Uber die Absorption divergenter" (1918). back
Note 157: Rutherford to Pettersson, January 9, 1928, GUB. back
Note 158: Karlik and Kara-Michailova, "Über die durch alpha-Strahlen erregte Lumineszenz" (1928); Karlik and Kara-Michailova, "Zur Kenntnis der Szintillationsmethode" (1928). back
Note 159: November 6, 1928, Karlik's file, Mitarbeiter/Assistenten, AÖAW. back
Note 160: Chariton and Lea, "Some Experiments Concerning the Counting of Scintillations" (1929), 336. back
Note 161: In a letter of June 23, 1928, Chadwick asked Meyer if he would facilitate Chariton's visit to Vienna. Chariton was in his way to Russia and because of visa restrictions, he was not allowed to cross Austria without a reason. "If the authorities are assured by a resident in Vienna of the purpose of his visit, they will be satisfied," as Chadwick explained to Meyer. "His [Chariton's] work has been on the counting and scintillations and I think" as Chadwick continued "you will be interested in what he has to say" (Chadwick to Meyer, June 23, 1928, AÖAW). However, it is interesting that in Chariton's case, Chadwick does not ask for permission but somewhat haughtily assumes that his student should be welcomed in Vienna. back
Note 162: Rona, How it Came About (1978), 20. back
Note 163: Kara-Michailova and Karlik, "Über die relative Helligkeit" (1929). back
Note 164: Karlik, "Uber die Szintillationsfähigeit" (1930); Karlik, "Untersuchungen zur Lumineszenz" (1930). back
Note 165: Blau and Kara-Michailova, "Über die durchdringende Strahlung" (1931). back
Note 166: Meyer to the Austrian Federation of University Women, September 26, 1933, AÖAW (in English).On October 26, 1935, Kara-Michailova wrote to Meyer from Girton College, Cambridge, to thank him for the pleasant years she spent at the Institute. She hoped for further collaboration in the future. (Kara-MIchailova to Meyer, October 26, 1935, AÖAW). back
Note 167: Kara-Michailova, "The Radioactivity of the Water Sources" (1960); Kara-Michailova and Kamburov, "Radiological and Hydrological Research" (1961), 109; Kara-Michailova, Nikolov, and Doitchinova, "The Radioactivity of Mineral Water-Springs" (1962). As Tsoneva-Mathewson, and the Rayner-Canhams argue, Kara-Michailova organized her own laboratory at the University of Sofia and many of the instruments were handmade. (Tsoneva-Mathewson; Rayner-Canham, M. and Rayner-Canham, G., "Elizaveta Kara-Michailova," (1997), 206). See also Nazarska, "Karamichailova, Elissabeta Ivanova" (2006); Stretenova, "Elizaveta Karamihailova (1897-1968)" (2003). back
Note 168: Lintner, "Berta Karlik, Nachruf" (1990), 306. back
Note 169: Karlik and Knaggs, Tables of Cubic Crystal Structures (1932); Karlik and Gilchrist, "Separation of Normal Longchain Hydrocarbons" (1932), 1992. back
Note 170: Pettersson, Report to the International Education Board, Part II, Box 56/923, RAC. back
Note 171: Kara-Michailova, "Helligkeit" (1929), 361. back
Note 172: Pettersson, Report to the International Education Board, April 1928, GUB. back
Note 173: Karlik to Pettersson, July 26, 1933, GUB. back
Note 174: Hughes, "Modernists with a Vengeance" (1998), 344. back
Note 175: Oliphant, "Working with Rutherford" (1984), 185–6. back
^top